What is quantum theory?
Quantum theory, or quantum mechanics, is the theoretical basis of modern physics that explains the nature and behavior of matter and energy on the subatomic level.
Quantum theory deals with subatomic phenomena while the theory of relativity deals with the macroscopic world. Together they form the basis of modern physics. Because they deal with different domains, this has led physicists to search for a so-called unified theory of everything.
Organizations in several countries have devoted significant resources to the development of quantum computing, which uses quantum theory to drastically improve computing capabilities beyond what is possible using today's classical computers.
The founding of quantum theory
In 1900, physicist Max Planck of Plank's constant fame, presented his quantum theory to the German Physical Society.
Planck had sought to discover the reason that radiation from a glowing body changes in color from red to orange and, finally, to blue as its temperature rises. He found that by assuming energy existed in individual units in the same way that matter does, rather than just as a constant electromagnetic wave -- as had been formerly assumed -- and was therefore quantifiable, he could find the answer to his question. The existence of these units became the first assumption of quantum theory.
Planck wrote a mathematical equation involving a figure to represent these individual units of energy, which he called quanta. The equation explained the phenomenon very well; Planck found that at certain discrete temperature levels (exact multiples of a basic minimum value), energy from a glowing body will occupy different areas of the color spectrum. Planck assumed there was a theory yet to emerge from the discovery of quanta, but, in fact, their very existence implied a completely new and fundamental understanding of the laws of nature.
Planck won the Nobel Prize in Physics for his theory in 1918, but developments by various scientists over a thirty-year period all contributed to the modern understanding of quantum theory.

The development of quantum theory
Quantum theory developed rapidly between its founding in 1900 through the end of the 1920s:
- In 1900, Planck made the assumption that energy was made of individual units, or quanta.
- In 1905, Albert Einstein theorized that not just the energy, but the radiation itself was quantized in the same manner.
- In 1924, Louis de Broglie proposed that there is no fundamental difference in the makeup and behavior of energy and matter; on the atomic and subatomic level either may behave as if made of either particles or waves. This theory became known as the principle of wave-particle duality: elementary particles of both energy and matter behave, depending on the conditions, like either particles or waves.
- In 1927, Werner Heisenberg proposed that precise, simultaneous measurement of two complementary values -- such as the position and momentum of a subatomic particle -- is impossible. Contrary to the principles of classical physics, their simultaneous measurement is inescapably flawed; the more precisely one value is measured, the more flawed will be the measurement of the other value. This theory became known as the uncertainty principle, which prompted Albert Einstein's famous comment, "God does not play dice."
The Copenhagen interpretation and the many-worlds theory
The two major interpretations of quantum theory's implications for the nature of subatomic particles are the Copenhagen interpretation and the many-worlds theory.
Copenhagen interpretation
Physicist Niels Bohr was the main contributor to the Copenhagen interpretation of the quantum theory, which asserts that a particle is whatever it is measured to be (for example, a wave or a particle), but that it cannot be assumed to have specific properties, or even to exist, until it is measured. This translates to a principle called superposition that claims that while we do not know what the state of any object is, it is actually in all possible states simultaneously, as long as we don't look to check.
The Copenhagen interpretation makes no claim as to the physical state of a particle while it is in a superposition state and it is primarily concerned with the underlying math of the wave function.
Double-slit experiment
The double-slit experiment is a practical example that helps illustrate superposition. It can show how a single photon can be in two places at once.
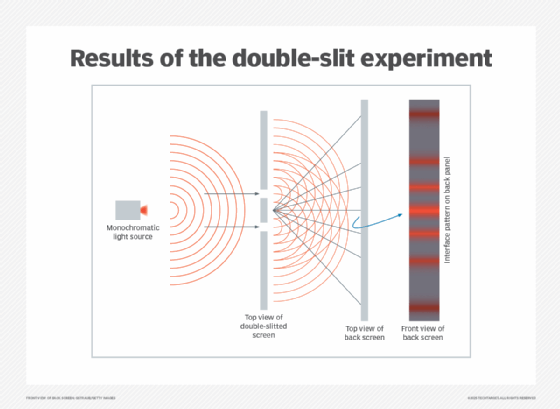
Famed physicist, Richard Feynman, said of the double-slit experiment that it "has in it the heart of quantum physics, in reality, it contains the only mystery." So, by understanding it, we can gain insight into how classical physics differs from quantum mechanics and start to understand quantum effects.
Schrodinger's cat
The Schrodinger's cat thought experiment was first used to show the seeming absurdity of the Copenhagen interpretation, but is now used as a quick way to explain it. In it, we have a living cat and place it in a thick lead box. At this stage, there is no question that the cat is alive.
We then put in a vial of cyanide that can be broken by a random quantum process (such as on the decay of a radioactive atom) and seal the box. We do not know if the cat is alive or if the cyanide capsule has broken and the cat has died.
Since we do not know, the cat is both dead and alive, it is in a superposition of states. It is only when we break open the box and see what condition the cat is that the superposition is lost, and the cat must be either alive or dead.
Many-worlds theory
The second interpretation of quantum theory is the many-worlds (or multiverse) theory. It holds that as soon as a potential exists for any object to be in any state, the universe of that object transmutes into a series of parallel universes equal to the number of possible states in which the object can exist, with each universe containing a unique single possible state of that object.
Furthermore, there is a mechanism for interaction between these universes that somehow permits all states to be accessible in some way and for all possible states to be affected in some manner. Stephen Hawking and Richard Feynman are among the scientists who have expressed a preference for the many-worlds theory.
Quantum theory's influence
Although scientists throughout the past century have balked at the implications of quantum theory -- Planck and Einstein among them -- the theory's principles have repeatedly been supported by experimentation, even when the scientists were trying to disprove them. Quantum theory and Einstein's theory of relativity form the basis for modern physics.
The principles of quantum physics are being applied in an increasing number of areas, including quantum optics, quantum chemistry, quantum computing, and quantum cryptography.
Quantum theory is important to the development of superconductors. New research is also being done to utilize quantum states to store energy, so-called quantum batteries. These developments could be used to help countries in a renewable energy transition.
Like any emerging technology, quantum computing offers opportunities and risks. Learn how quantum computing compares to classical computing and explore quantum key distribution for exchanging encryption keys. Read about the qubit, the basic unit of information in quantum computing.
