heat
What is heat?
Heat is the transfer of thermal energy from one physical system to another system or from one region in a physical system to another region. A system, in such an instance, might be a mug of coffee, room full of air, cast-iron frying pan, mountain lake, piece of scrap metal, or any number of other physical objects or substances, including an energy source such as a campfire or the sun.
Thermal energy tends to move from an object or region with a higher temperature to one with a lower temperature. For example, if Object A and Object B are connected, and Object A has a higher temperature than Object B, heat moves from Object A to Object B, causing Object A's temperature to decrease and Object B's temperature to increase. This also means that Object A's average kinetic energy is decreasing and Object B's average kinetic energy is increasing.
Heat plays a critical role in the lives of humans and other organisms -- all of which are systems. It gives us comfort by warming our skin, while helping to maintain optimal body temperatures. For humans, heat helps in preparing foods, warming homes and manufacturing goods. Heat generated by sunlight helps plants to mature and develop fruit, and heat also helps to shape local and global weather patterns.
The term heat is sometimes confused with the term temperature, although they have distinctly different meanings. Temperature is the measure of the average kinetic energy of a system's atomic particles, whereas heat is concerned with the transfer of thermal energy, which results from the kinetic energy generated by moving particles. Temperatures can be measured in Fahrenheit, Celsius or kelvin. Heat can be measured in joules (J), calories (cal) or British thermal units (BTU):
- Joule. The standard unit of heat in the International System of Units. One joule is equal to 1 newton multiplied by 1 meter (J = N ⋅ m). A joule can also be expressed by the formula J = kg ⋅ m2 ⋅ s-2, where kg is kilograms and s is seconds.
- Calorie. The amount of thermal energy transfer required to raise the temperature of 1 gram of pure liquid water by 1 degree Celsius at sea level. One calorie is equal to about 4.18 J.
- BTU. The amount of thermal energy transfer required to raise the temperature of 1 pound of pure liquid water by 1 degree Fahrenheit at sea level. One BTU is equal to about 1,055.06 J.
When two systems reach the same temperature -- that is, their kinetic energy evens out -- they are said to be in a state of thermal equilibrium. For example, if you put an ice cube into a cup of hot tea, the tea's heat transfers to the ice cube, causing the tea to cool and the ice cube to melt. The tea and melted water eventually reach the same temperature, resulting in a state of thermal equilibrium.
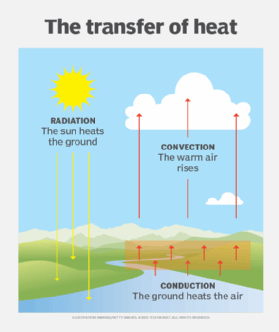
Types of heat transfer
The way in which thermal energy is transferred within or between systems depends on the type of system and its physical state. Heat transfer can occur in one of three ways:
- Conduction. Heat by conduction takes place when two systems are in direct contact and the temperature of one system is higher than the temperature of the other. Because temperatures tend to equalize across connected physical systems, thermal energy travels from the warmer system to the cooler one until both systems reach thermal equilibrium. For example, if a blacksmith immerses a hot iron in a tub of cold water, the iron's heat is transferred to the water, causing the iron to cool and the water to heat. If the iron is left in long enough, the iron and water eventually reach the same temperature.
- Convection. Heat by convection occurs when the motion of a liquid or gas carries thermal energy from a warmer region within a system to a cooler region. The liquid or gas circulates, with the warmer substance moving upward and the cooler substance moving downward. A good example of convection is a room that contains a wood-burning stove. As the stove heats the air around it, the warm air rises toward the ceiling, and the cool air near the ceiling sinks toward the floor, causing the air to circulate around the room. Heat convection, along with conduction, is believed to take place inside the Earth's mantle, transferring thermal energy from the outer core to the crust.
- Radiation. Heat by radiation occurs when an energy source transfers thermal energy to another system via electromagnetic (EM) waves. A common example of radiated heat is infrared (IR) energy as it strikes a surface. For example, sunlight emits IR radiation that warms the Earth when it hits the planet's surface, much like a campfire warms the bodies of people sitting around it. Radiation does not require an intervening medium, such as the Earth's atmosphere. EM waves can travel through a vacuum, which is why the sun is able to warm the Earth.
Learn how to calculate data center cooling requirements, and explore liquid cooling vs. air cooling in the data center.
