electron
What is an electron?
An electron is a negatively charged subatomic particle that can be either bound to an atom or free (not bound). An electron that is bound to an atom is one of the three primary types of particles within the atom -- the other two are protons and neutrons.
Together, protons and electrons form an atom's nucleus. A proton has a positive charge that counters the electron's negative charge. When an atom has the same number of protons and electrons, it is in a neutral state.
Electrons are unique from the other particles in multiple ways. They exist outside of the nucleus, are significantly smaller in mass and exhibit both wave-like and particle-like characteristics. An electron is also an elementary particle, which means that it is not made up of smaller components. Protons and neutrons are thought to be made up of quarks, so they are not elementary particles.
Shells, subshells and orbitals
In the early days of atomic study, scientists believed that an atom's electrons circled the nucleus in spherical orbits at specific distances, much like planets circle a sun. In this model -- referred to as the Bohr model -- the orbits furthest from the nucleus contain the greatest amount of energy. When an electron jumps from a higher energy orbit to a lower energy orbit, the atom releases electromagnetic radiation.
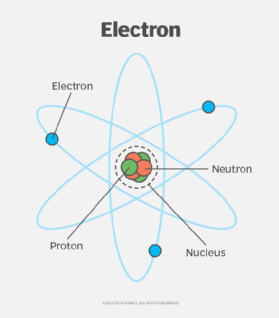
The Bohr model is no longer thought to be accurate, particularly as it pertains to how the electrons orbit the nucleus. While the model can still be useful in understanding the basics of electron distribution and different energy levels, it fails to consider the complexity of that distribution and how electrons inhabit the space around the nucleus, according to current quantum theory.
Electron movement is determined by calculating the probability of finding electrons in specific regions within the space that surrounds the atom's nucleus -- rather than by assuming fixed trajectories. The mathematically defined regions are based on three structural patterns:
- Shells. The concept of a shell originates with the Bohr model, although the theory around shells has evolved. Physicists now believe that a shell is a region of probability surrounding the nucleus. An atom can contain up to seven electron shells, depending on the type of atom. The shells exist at different levels around the nucleus. Shells furthest from the nucleus have the highest amounts of energy and those nearest have the lowest. Each shell is limited to a specific number of electrons, depending on its level and the configuration. A shell can contain one or more subshells, and a subshell can contain one or more orbitals.
- Subshells. A subshell is a collection of one or more orbitals of a specific type. There are four types of orbitals and subsequently four types of subshells -- designated as s, p, d and f, depending on their orbitals. An s subshell contains one s orbital, a p subshell contains three p orbitals, a d subshell contains five d orbitals, and an f subshell contains seven f orbitals. It has also been theorized that an atom can support a g subshell that contains nine g orbitals.
- Orbitals. An orbital is a specifically shaped region of space around the nucleus where an electron is most likely found. In other words, it is the region with the highest probability (over 90%) of containing the electron as it travels around the nucleus. An orbital might be shaped like a sphere (s orbital), a dumbbell (p orbital) or a more complex shape (d and f orbitals). Whatever its shape, an orbital can include a maximum of two electrons.
An atom's shells are numbered consecutively, starting at the nucleus and working out. A shell's number is often referred to as its n value. For example, the third shell might be referred to as n=3 or 3n. Letters are also sometimes used to refer to the shells. These include K, L, M, N, O, P and Q, again starting from the nucleus and working out. For instance, the third shell might be referred to as the M shell or 3m.
Each shell contains one or more specific types of subshells, which determine the maximum number of electrons that the shell can contain. For example, the first shell (K) contains a single s subshell that includes only one s orbital. As a result, the maximum number electrons that the shell can contain is two. This means that an atom that has only a K shell is limited to two electrons. Only two elements, hydrogen and helium, have a single shell. Hydrogen contains only one electron and helium contains two.
The subshell/orbital configuration varies from one shell to the next, growing more complex until the fifth shell, at which point the complexity starts to taper off. For instance, the second shell (L) includes an s subshell and a p subshell. The s subshell contains one s orbital, and the p subshell contains three p orbitals. This means the shell can support up to eight electrons.
However, an atom with an L shell also contains a K shell. In fact, the L shell will start filling up after the K shell is filled. This means that an atom with an L shell can support up to 10 electrons because of the presence of both the K and L shells. For example, lithium and neon contain both K and L shells. A lithium atom has only three electrons, two in the K shell and one in the L shell, but a neon atom has 10 electrons, two in the K shell and eight in the L shell.
In general, this same pattern continues for all seven shells, with the inner shells filling up with electrons before the outer shells. However, this is only a tendency. Electrons gravitate toward the most stable configuration, which is usually the inner shells, but it's also possible for an outer shell to start filling up with electrons before the lower shell is completely full.
Regardless of the order in which shells fill with electrons, the shells themselves determine the maximum number of electrons they can support based on their subshells and orbitals. All but the first shell includes a p subshell, only the third through sixth shells contain d subshells, and only the fourth and fifth contain f subshells. All seven shells include an s subshell.
Electrons and electricity
In electrical conductors, current flows as a result of electrons jumping from atom to atom as they move from negative to positive electric poles. In semiconductor materials, current also results from electron movement, however, the movement is based on electron deficiencies in atoms. An electron-deficient atom in a semiconductor is called a hole. In this case, the current moves from positive to negative electric poles.
The charge of a single electron is referred to as the unit electrical charge. It carries a negative charge that is equal to but opposite the positive charge on a proton or hole. However, the amount of electrical charge is usually not measured on a single electron because that amount is so small.
Instead, the standard unit of electrical charge is the coulomb (symbolized by C). A coulomb contains about 6.24 x 1018 electrons. An electron's charge (symbolized by e) is about 1.60 x 10-19 C. The mass of an electron at rest (symbolized by me) is approximately 9.11 x 10-31 kilograms (kg). If electrons are accelerated to nearly the speed of light, as in a particle accelerator, they will have greater mass because of relativistic effects.
See also: Table of Physical Units, clean electricity, electrical pollution, electric grid, volt per meter, electron rest mass, ion, electric charge
