dielectric constant
What is dielectric constant?
The dielectric constant of a substance or material is a measure of its ability to store electrical energy. It is an expression of the extent to which a material holds or concentrates electric flux.
Mathematically, dielectric constant is the ratio of a material's permittivity to the permittivity of free space. This is why it is also known as relative permittivity. It is the electrical equivalent of relative magnetic permeability.
More about dielectric constant
The value of the dielectric constant represents the ratio of the capacitance of the capacitor whose test material is the dielectric to the capacitance of a capacitor whose dielectric is vacuum (or air).
It is mathematically expressed as the following: k = ϵ/ϵ0
The variables in this equation are defined as follows:
- ϵ is the permittivity of the substance;
- ϵ0 is the permittivity of free space; and
- k (the Greek letter kappa) is a unitless and dimensionless quantity since it is the ratio of two like entities (permittivity). Different materials have different dielectric constants, indicating the extent to which they can store an electrical charge.
As the dielectric constant increases, the electric flux density increases (if all other factors remain unchanged). This property enables objects of a given size, such as metal plates, to hold large quantities of electric charge for long periods of time.
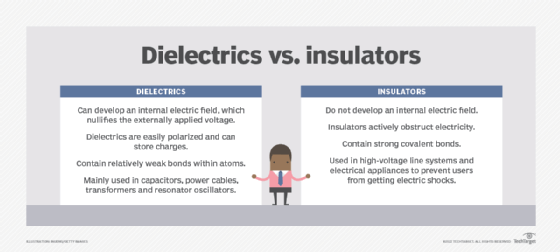
A high dielectric constant is not necessarily desirable. Generally, substances with high dielectric constants break down more easily when subjected to intense electric fields compared to materials with low dielectric constants (see Figure 1).
Dielectric materials
A dielectric material has weak electrical conductivity but can store an electrical charge. When it is placed in an electrical field, no electric current flows within it. This is because the material does not have loosely bound (free) electrons that drift through it. This property is what differentiates electrical insulators from electrical conductors.
Instead, the positive and negative charges within the dielectric are both displaced -- the positive charges in the direction of the electric field and the negative charges in the opposite direction. This phenomenon of charge separation, known as polarization , reduces the electric field in the dielectric.
When a dielectric is inserted between the plates of a parallel-plate capacitor, it increases the capacitor's capacitance, i.e., its ability to store opposite charges on each plate. However, this doesn't happen when the capacitor plates are separated by a vacuum. This is why the dielectric constant value of any dielectric material is always greater than the dielectric value for a vacuum, which is one (1).
Dielectric constant of common materials
Dry air has a low dielectric constant. It can undergo dielectric breakdown , a condition in which the dielectric suddenly begins to conduct electrical current. However, the breakdown is not permanent because when the excessive electric field is removed, air will return to its normal dielectric state. Some other materials also exhibit this property, which prevents them from incurring permanent damage.
The dielectric constant of air is very close to the dielectric constant of vacuum. This is why neither vacuum nor air increase the capacitance of a capacitor. On the other hand, solid dielectric substances such as polyethylene or glass, which have a higher dielectric constant, can sustain permanent damage as the electrical current increases and they lose their dielectric properties.
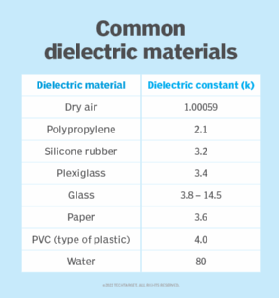
Figure 2 shows the dielectric constants of common dielectric materials.
Factors affecting the dielectric constant
The dielectric constant of any material is affected by temperature and current frequency. Other factors also affect the value.
Temperature
As mentioned earlier, charge separation or polarization affects the electric field in a dielectric material. Temperature changes affect polarization and consequently, the dielectric constant. For instance, when water is heated from 0o C to 100o C, its dielectric constant falls from 80 to 55. Thus, the dielectric constant is inversely proportional to temperature.
Applied voltage
In the presence of a direct current voltage , the value of the dielectric constant decreases. Contrastingly, when an alternating current voltage is applied, the value of the dielectric constant increases.
Frequency
The frequency of the applied voltage also affects the dielectric constant. As the frequency increases, the dielectric constant becomes non-linear and its value decreases more rapidly as the frequency increases. At high frequencies, electric power loss increases. This is one reason why materials with low dielectric constant values are preferred for high frequency applications.
Humidity
Humidity is inversely proportional to dielectric constant. As the humidity or moisture of the material increases, its dielectric constant decreases, affecting its dielectric strength.
Applications of dielectric constant
The dielectric constant is a crucial parameter to consider when selecting the dielectric material for a capacitor and in any circumstance where a material is required to introduce capacitance into an electrical circuit or printed circuit board (PCB).
The dielectric value -- and by extension the dielectric material it represents -- also has numerous other applications, including the following:
- radio frequency transmission lines and communications;
- energy storage devices;
- electrical substation equipment; and
- transformers and rheostats.
See also: picofarad per meter, flash storage, resistive RAM, floating gate transistor, inductor, ultracapacitor, transducer and liquid immersion cooling.
