What is the FDA (U.S. Food and Drug Administration)?
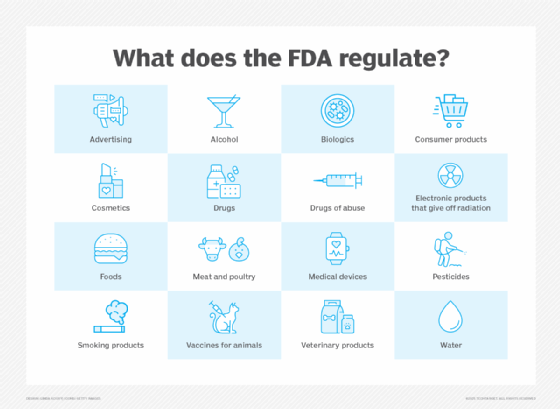
The FDA (U.S. Food and Drug Administration) is an agency within the U.S. Department of Health and Human Services (HHS) responsible for protecting public health by ensuring the safety, efficacy and security of human and veterinary drugs, biological products, medical devices, food supply, cosmetics, and products that emit radiation.
It also plays a critical role in advancing public health by facilitating innovations that make medicines and foods more effective, safer and more affordable. Additionally, it regulates the manufacturing, marketing and distribution of tobacco products.
Guides to the FDA approval processes and guidelines for medical and radiologic devices are available on the agency's website.
History of the FDA
The FDA defines itself as the country's oldest consumer protection agency. It traces its origins to the U.S. Patent and Trademark Office in 1848 and the creation of the U.S. Department of Agriculture in 1862, which carried out the Patent Office's duties during chemical analyses of agricultural products. The FDA's regulatory functions started with the 1906 passing of the Pure Food and Drug Act.
Over the years, the FDA's responsibilities have expanded significantly to encompass a broader range of products and technologies. Today, it regulates $1 trillion worth of products annually -- including most foods and beverages, infant formula, pet foods, tobacco products, microwave ovens, prescription and over-the-counter drugs, vaccines and much more.
While the FDA might be most closely associated with activities like drug approvals and food safety recalls, it also plays a key role in regulating many healthcare technologies.
FDA regulation of medical devices
As it relates to the healthcare industry, one of the FDA's functions is to protect the public from radiation produced by electronic medical and consumer devices.
All products that give off radiation, such as lasers, X-ray systems and ultrasound equipment, must be preapproved by the FDA's Center for Devices and Radiological Health (CDRH) before they can go to market. The CDRH categorizes medical devices into three classes based on risk:
- Class I (Low risk). Devices subject to general controls, such as elastic bandages, wellness wearables and examination gloves.
- Class II (Moderate risk). Devices requiring general and special controls, including powered wheelchairs and infusion pumps.
- Class III (High risk). Devices that support or sustain human life, requiring premarket approval, such as implantable pacemakers and breast implants.
After the CDRH recognizes that a product was safely manufactured, state agencies supervise the use of that product within their jurisdictions.
Digital health and mobile medical applications
With the proliferation of digital health technologies, the FDA has provided guidance on mobile medical applications (MMAs). The agency exercises enforcement discretion for low-risk MMAs that promote general wellness or assist in self-managing diseases without providing specific treatment suggestions.
However, MMAs that function as medical devices, such as those transforming a consumer smartphone into a regulated medical device, are subject to FDA oversight and must comply with applicable regulatory requirements.
As the use of AI and machine learning has surged, the FDA has taken a more active role in regulating its applications in healthcare. In 2024, the FDA issued guidance intended to simplify the regulation and approval of AI-enabled medical devices. However, the agency has acknowledged that the fast-evolving landscape means it will likely continue to iterate on such guidance.
Cybersecurity in medical devices
Recognizing the growing threat of cyberattacks on medical devices, the FDA has issued guidance to enhance cybersecurity measures. Medical device manufacturers are encouraged to incorporate cybersecurity considerations throughout the device lifecycle, including during design, development and maintenance.
The FDA's guidance outlines recommendations for managing cybersecurity risks and emphasizes the importance of maintaining device functionality and patient safety in the face of potential cyberthreats.
The FDA's evolving regulatory framework reflects its commitment to adapting to technological advancements while ensuring public health and safety. Staying informed about FDA regulations empowers stakeholders to better navigate the complex landscape of medical device development, digital health innovations and cybersecurity challenges effectively.
The regulation of medical devices encompasses various stages, from development through to post-market monitoring. It is important to note that the specific regulatory pathways for these devices are determined by their classification. Learn about the regulatory pathways for medical devices.





