
Getty Images
Warp Speed COVID-19 Clinical Trials Boost Future Vaccine Development
Vaccine development is generally a time-consuming process, but the unprecedented speed of COVID-19 clinical trials will have implications across the pharmaceutical industry.
Discovering and developing a novel vaccine candidate is a lengthy process. Even after the vaccine is created and tested for safety, licensing and manufacturing draw out the process making it years before it is available to the general public.
Based on typical FDA processes, that time could be more than ten years, as the regulatory body requires vaccines to undergo at least three phases of clinical trial development before applying for approval.
And some new vaccines may even need to undergo a fourth trial stage to validate efficacy and assess the vaccine’s durability after it has been distributed. This allows researchers to understand the effects of the vaccine in the general population and calculate the longevity of the vaccine. Will individuals need a booster shot? Is the vaccine effective for five years? Ten years?
But those inside and outside of the pharmaceutical industry overwhelmingly agree that we don’t have that time in the face of a global pandemic.
There have been nearly 21 million cases of COVID-19 around the world, according to data from Johns Hopkins University. And that number continues to rise as most countries face testing shortages, hospital capacity challenges, and even resurgences of the virus after declining numbers.

Pharmaceutical companies and governments are working on a COVID-19 vaccine at an unprecedented speed. In the US, for example, the White House’s Operation Warp Speed is bringing together public and private stakeholders to deliver 300 million doses of a safe and effective COVID-19 vaccine by January 2021.
But the unprecedented speed of COVID-19 vaccine trials has many outside of the industry questioning the efficacy of the end product. Leaders at organizations across the industry including the FDA, Department of Health and Human Services (HHS), National Institutes of Health (NIH), and Centers for Disease Control and Prevention (CDC) have repeatedly assured the public that the final approved coronavirus vaccine will have undergone the same scrutiny and meet the same standards as any other vaccine.
Yet steps the industry is taking to accelerate COVID-19 vaccine development will undoubtedly shift future vaccine research and development for other novel drugs.
“We are facing a pandemic, the likes of which we haven’t seen in at least one hundred years,” James Mayne, PhD, vice president of science and regulatory advocacy at PhRMA told PharmaNewsIntelligence. “We need the energy and expertise that’s been going into finding real, workable solutions. It’s absolutely where we should be dedicating our time and energy right now.”
Accelerating the vaccine development process could very well lead to not only one, but many viable options.
“We’re taking multiple shots on goal. Multiple shots on goal leads to multiple shots in goal. And in vaccines, this leads to multiple shots in arms,” Mayne continued. “We’re going to have the good fortune of having a couple of different successful vaccines of different types.”
But how are pharmaceutical companies and government agencies bypassing years of clinical trial work to create a safe and effective vaccine that people will trust?
Clinical Trials for Vaccine Development
Vaccine clinical trials differ from other drug clinical trials in one critical way: vaccines go into healthy people.
“With the moral principle of medicine to do no harm, there is a lot of scrutiny around vaccines,” explained Barry Bloom, PhD, research professor at the Harvard T.H. Chan School of Public Health. “Drugs go into people who are sick and often have life-threatening illnesses. The margin for suffering adverse effects is greater in therapeutic trials than it is in a vaccine trial.”
But monitoring safety and adverse effects is still a priority in all stages of clinical trial development.
Phase 1
The first phase of a clinical trial is also the first time the vaccine is injected into humans. These are small studies, usually containing only 20 to 100 people, according to the CDC.
The goal of phase 1 clinical trials is to understand basic safety, monitoring for adverse reactions to the vaccine.
“Phase 1 is safety. You put 100 people and look for really serious consequences from the injection. You don’t expect most vaccines to fail at that stage but if you do, you shut them down,” explained Bloom.
Phase 2
Phase 2 trials are larger, involving hundreds of participants.
“In phase 2, you’re looking at a product like antibodies to see if the vaccine has the potential for a protective immune response. If it didn’t extend at that point, it is not going to go forward,” elaborated Bloom.
Safe dosing is tested in a larger population to ensure the desired immunological response is triggered. Researchers also monitor for adverse outcomes, always ensuring the vaccine is safe.
Phase 3
In the next stage, several hundred to several thousand participants are divided, typically, into two groups. One group receives the vaccine and the other does not. The goal at this phase is to compare the two groups and establish if the vaccine if effective at preventing the disease.
Researchers also continue to monitor the frequency of adverse events and the vaccine’s safety.
“You always want to be monitoring safety, even to make sure you aren’t seeing any type of low incidence events that you might have been able to detect in clinical studies if you have 30,000 participants,” Mayne elaborated.
Having a large group of participants ensures the statistical significance and efficacy of the vaccine. Statisticians calculate the minimum number of individuals who must participate for the sample population to be representative of the larger population.
“Getting really solid, statistically significant, well designed, representative of the population data for vaccines is absolutely essential for two reasons,” Bloom articulated. “One is that you really have to be sure you’re doing no harm. The second is that the consequences, in a world of anti-vaccine movements and social media with increasing skepticism of the government, is that any vaccine that does harm is going to do harm to all vaccines.”
After the completion of a successful phase three trial, the vaccine can seek licensure and approval from the FDA to roll out into the larger population.
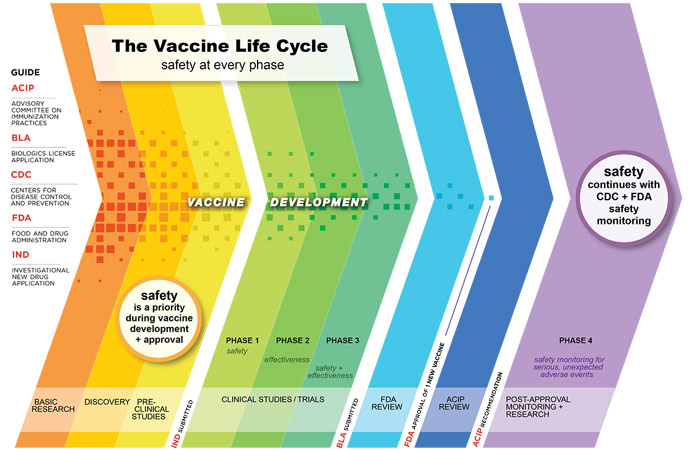
The Additional Phase 4 Trial
The final clinical trial phase is infrequently completed. Phase 4 trials monitor the safety of a vaccine after it has been approved for public use. In this type of study, the Advisory Committee on Immunization Practices continuously monitors patients who have received the vaccine.
“Usually its one to two years after a new vaccine where every physician, every hospital, if they see an adverse event, has to report it to the local and state authorities and ultimately the CDC,” Bloom explained.
Once in the marketplace, the vaccine is monitored for longevity: how long is it effective in patients? What is its durability? Will it need a booster over time?
“Any of these new vaccines, we don’t know how long they’ll last,” Bloom continued. “That’s only going to be known when you do phase 4 clinical trials. Once the vaccine is found to be safe and effective and distributed, we really need to look for the long-term consequences of this vaccine.”
A phase 3 trial cannot last indefinitely. After its completion, researchers feel confident the drug is safe and effective to roll out into a larger population.
“They’ll continue to learn as they move out into the marketplace and do what we can to optimize the formulation or the performance of the products,” said Mayne.
Overall, phase 4 trials allow researchers to understand the longer-term consequences of the vaccine while still allowing patients to have access to the vaccine after it's proven to be safe.
Getting to Phase 4 As Quickly As Possible
The COVID-19 vaccine development process is being accelerated. Compared to the years it has taken other vaccines to come to market, experts predict a coronavirus vaccine could be ready as early as January 2021.
“When we have a pandemic that is causing mayhem, both medically and economically, there’s tremendous incentive to get a successful vaccine out as rapidly as possible,” Mayne articulated.
Fortunately, the pharmaceutical industry has “all of the tools already in its tool chest and was ready to rise rapidly to this challenge,” Mayne emphasized.
One of these tools is the ability to identify the virus’ DNA sequence.
Traditionally, upon identification of a novel virus, the virus had to be taken apart piece by piece to understand its different components. Researchers would then identify which elements of the virus triggered an immune response, purify that agent, and test it in animals.
Advancements in DNA technology expedite the process. The virus’ messenger molecule, RNA, can be extrapolated from the DNA sequence. This allows researchers to target a specific protein that is carrying the virus into the human body.
“It makes the front-end of the research development process go very quickly,” Mayne said.
In February, researchers from Korea isolated the novel coronavirus’ genetic sequence, less than two months after the virus first emerged in Wuhan, China.

Accelerating the front-end vaccine development is not enough, though. Speeding up the manufacturing process will also help to distribute the vaccine to the greater population quicker, which is key to getting to the phase 4 stage faster.
“To make a vaccine you have to build a factory. You have to set up supply chains and you have to have a cold chain,” Bloom explained. “Usually, this takes a lot of time.”
What’s happening differently in coronavirus vaccine development, though, is that manufacturing is already underway before clinical trial testing has concluded. These companies are taking risks beginning manufacturing before the vaccine is proved safe and effective. But this could have a high reward because once the vaccine is approved, it can immediately be dispensed.
“The speed is really coming from the ability to engage the issue and come up with viable candidates. This is all on the front-end which most people have seen in the press. But everyone should be assured as well that on the backend, all of the manufacturing preparation that’s going on is not cutting corners,” Mayne assured.
And collaboration across the pharmaceutical industry is enabling the pharmaceutical company to accelerate processes on both the front- and back-end of vaccine development.
“There is a tremendous amount of collaboration and engagement across the industry in the span of just of few months. This has brought us to the place where we have phase three studies ongoing for multiple vaccines,” furthered Mayne. “Companies are in regular contact with opportunities for them to get together and share their experience and their data. They’re building real databases that people can use even outside of the companies to learn more about the virus and learn about some of the ways for us to try and treat it.”
Logistically, no single company has the capacity to develop and manufacture a vaccine for the entire world. Collaboration is essential.
“Every one of those companies is engaged in trying to solve this problem one way or another,” Mayne continued. “They’ve got all these players and they can go very quickly to bring forward candidate vaccines. They don’t have to go out and hire people or build factors to do these types of things.”
Future Impact on Vaccine Development
Despite assurances from HHS, the CDC, NIH, and FDA that no corners will be cut, many are hesitant a coronavirus vaccine will be dangerous.
“One of the things that we are certainly not cutting corners on is the quality of clinical information that informs a regulatory decision on the product in the marketplace,” Mayne emphasized. “I want to do everything I can to put people at ease over this next year that these vaccines when compared to other vaccines, are the same in terms of safety. They’re doing studies with the same numbers of patients and looking at the same clinical endpoints.”
Every new product the FDA reviews, including vaccines, starts the approval process from step one.
Safety is not a concern, the experts say. A vaccine will not be dispensed without proper and standard safety and efficacy standards being met. But one concern remains: Will people trust this and take the vaccine?
“My biggest worry is that we will have adequate vaccines with appropriate safety, but people won’t take them. And they won’t take them in the mass numbers to develop herd immunity,” said Bloom.
The next step for the pharmaceutical industry will be to educate the population, reassuring everyone that the vaccine is safe and effective. Otherwise, the expedited vaccine development will be moot.
“We, as a society, need to learn from this so we are better prepared next time around beyond types of preventive measures and countermeasures,” Mayne concluded.
Ending the coronavirus pandemic and preparing the world for the next pandemic will require continuous collaboration across the pharmaceutical industry. And the trust seen within the industry must expand to the general public.



