Ca-ssis/istock via Getty Images
Understanding artificial womb technology for preterm infants
Artificial womb technology may present an opportunity for better outcomes among preterm infants; however, it also comes with many challenges.
The challenges of preterm birth are well understood by maternal health and pediatric professionals. While some factors that increase the risk of preterm birth can be addressed to minimize the potential of early labor, other factors are unavoidable. Healthcare professionals have implemented certain techniques, such as intensive care and corticosteroid medications, to address the complications of preterm birth; however, sometimes these techniques fail to deliver the care that additional gestational time would provide. Artificial womb technology is being researched as a potential tool for preterm infants to allow for further development and mimic the womb environment.
Preterm Birth
The Centers for Disease Control and Prevention (CDC) defines preterm birth as birth before 37 weeks of gestation. According to the organization, roughly 10% of infants born in the US in 2022 were born preterm.
Although preterm birth rates increased by 4% from 2020 to 2021, rates declined by 1% from 2021 to 2022. Across all races, the rate of preterm birth in 2022 was roughly 10.4%; however, rates among certain races were higher than the national average. For example, preterm birth rates among Black women were 14.6%, while the rates among White and Hispanic women were 9.4% and 10.1%, respectively.
Multiple factors are associated with an increased risk of preterm birth, including demographic or social factors, pregnancy and medical conditions, and behavioral characteristics.
The risk associated with preterm birth presents significant challenges for infants as the last weeks of gestation are often when the brain, lungs, and liver finish developing. Preterm infants are at a greater risk of death and disability. The CDC estimates that 14.8% of infant deaths in 2021 were linked to infants with preterm birth and low birth weight. Additionally, preterm infants who do survive often have breathing problems, feeding difficulties, cerebral palsy, developmental delay, vision problems, and hearing problems.
The challenges posed by preterm birth may be addressed through the use of artificial womb technology.
Artificial Womb Technology
Artificial womb technology, also known as ectogenesis, refers to the development of an external system that can provide an environment similar to the mother's womb to support the growth and development of a fetus outside the body. While this technology has been a topic of speculation and research for many years, artificial wombs are still in the experimental stage and have not been widely used in clinical settings.
According to the Massachusetts Institute of Technology (MIT) Technology Review, artificial wombs are experimental medical devices that are biobags filled with fluid that can theoretically hold a premature infant while they continue developing.
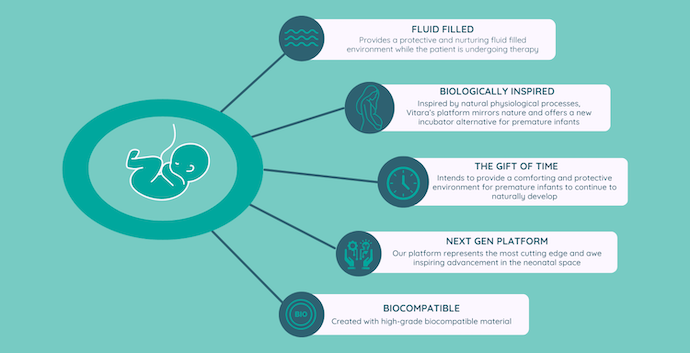
While artificial womb technology has many potential benefits, including mimicking the womb, reducing infection risk, and extending the gestational period, the primary goal of developing it is to provide a nurturing environment for premature babies who are born before they have fully developed and are unable to survive independently.
As previously mentioned, lung development is completed in the final few weeks of gestation; however, incomplete lung development significantly impacts survival in premature babies. Theoretically, premature babies can be placed in an artificial womb with lab-made amniotic fluid to mimic the environment they would have developed in if delivered at full term. The MIT Technology Review also notes, “Neonatologists would insert tubes into blood vessels in the umbilical cord so that the infant’s blood could cycle through an artificial lung to pick up oxygen.”
For example, Vitara Biomedical is working on the EXTrauterine Environment for Newborn Development (EXTEND). According to the MIT Technology Review, EXTEND is the artificial womb technology that is farthest along. The device is being tested in lamb fetuses and has had positive results.
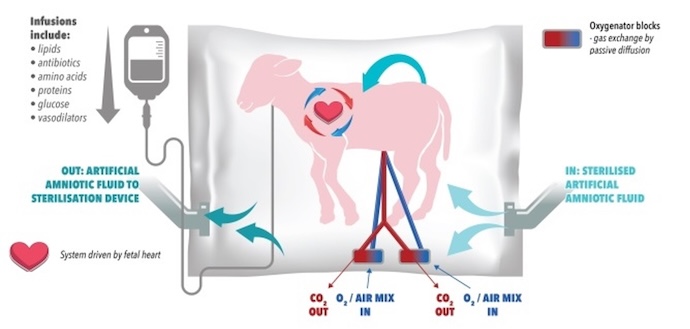
In addition to EXTEND, scientists in Australia and Japan are collaborating on artificial womb EVE therapy. The research is a collaborative effort between researchers at the Women and Infants Research Foundation, the University of Western Australia, and Tohoku University Hospital in Japan.
Below is an image of the EVE technology.
Another tool is being developed in Europe through the Perinatal Life Support project. Additionally, Canadian researchers are working on another artificial womb, focusing on piglets instead of lambs.
Research Challenges
Despite the potential utility of artificial womb technology, there are multiple research challenges posed by artificial womb technology.
- Replicating the complex biological processes: The womb provides a highly intricate and dynamic environment for fetal development, involving interactions between the mother and the fetus. Replicating these processes accurately in an artificial womb is a significant scientific and technical challenge.
- Mimicking placental function: The placenta plays a vital role in providing oxygen, nutrients, and waste removal for the developing fetus. Creating an artificial placenta capable of adequately performing these functions is a complex task that researchers are actively investigating.
- Fluid and circulatory systems: Developing a functional circulatory system that can deliver oxygen and nutrients to the fetus is a significant hurdle. Replicating the complex fluid dynamics and regulatory mechanisms of blood flow within the artificial womb is a considerable challenge.
- Maintaining a sterile environment: In a traditional womb, the fetus is protected from external pathogens and infections. Creating a sterile environment within an artificial womb and preventing the risk of contamination or infection is crucial but challenging.
- Supporting organ development: The development of vital organs, such as the lungs, brain, and immune system, is a complex process that occurs in coordination with the mother's body. Replicating the necessary conditions and interactions to support proper organ development in an artificial womb is a significant obstacle.
- Cost and scalability: Developing artificial womb technology that is both cost-effective and scalable for widespread use is a significant challenge. Achieving affordability and accessibility while maintaining safety and effectiveness is crucial for the technology's practical implementation.
Although researchers are working toward addressing these challenges, human testing may still be far off. Alan Flake, who worked alongside Marcus Davey to invent EXTEND at the Children’s Hospital of Philadelphia (CHOP), said the company planned on submitting an investigational device last year; however, he declined to publicly discuss the details of the device’s advancements. “To greenlight a trial, FDA officials need to be convinced that babies who try EXTEND are likely to benefit from the system and that they’ll fare at least as well as babies who receive the current standard of care,” stated the MIT Technology Review.
Beyond proving the benefits of treatment, researchers will also have to work out the logistics of human trials. From delivery to inserting the tubes into the umbilical cord and transferring the infant into the fluid-filled container, there is a large margin for error.
There are also multiple ethical considerations when it comes to artificial womb technology. Questions about the potential separation of the mother and the fetus, the long-term psychological and emotional effects on the child, and the broader societal implications need to be carefully addressed.
“The most challenging question to answer is how much unknown is acceptable,” said An Massaro, FDA’s lead neonatologist in the Office of Pediatric Therapeutics, at a committee meeting in September 2023.
George Mychaliska, a pediatric surgeon at the University of Michigan, told the MIT Technology Review that human trials would likely start on infants born at 22 or 23 weeks. At that point, no effective treatments are available.
“These babies are not a hopeless case. They very much can survive. They very much can thrive if you are managing them appropriately,” Brady Thomas, a neonatologist at Stead, considered in a statement to the MIT Technology Review. “Are you really going to make that much of a bigger impact by adding in this technology, and what risks might exist to those patients as you’re starting to trial it?”
As research progresses and technology advances, engaging in ongoing discussions and ethical debates will ensure that artificial womb technology is developed and utilized responsibly and beneficially for both infants and society overall.