
Getty Images
Understanding Implications of Pharmacogenomic on Prescribing
Pharmacogenomic research can enhance personalized medicine, optimize drug efficacy, improve safety, reduce treatment costs, and inform research and development.
Pharmacogenomic research explores how specific genes or genetic variations can impact drug responses and treatment outcomes. While not a standard of care, pharmacogenomic testing can significantly impact prescribing and medication safety. Studies in this area impact personalized medicine, drug efficacy, medication safety, overall treatment costs, and research and development.
“Pharmacogenetic-based variation can influence either the pharmacokinetic properties of the drug in an individual, such as the rate of absorption, distribution, metabolism, or elimination, or pharmacodynamic properties, such as how well a drug binds to a receptor site, thus making the drug available in each individual,” explains CVS Health.
In this article, PharmaNewsIntelligence explores the critical implications of pharmacogenomic research and testing on prescribing practices.
Personalized Medicine
One of the most significant implications of pharmacogenomics (PGx) is its ability to enhance precision or personalized medicine. Personalized medicine is a healthcare approach that uses a specific patient’s needs and molecular profile to match them to the appropriate treatment or therapy.
As personalized medicine becomes an increasingly popular field of study and healthcare, researchers and clinicians have discovered that effective personalized treatment hinges on a comprehensive understanding of the patient's molecular profile.
This is where pharmacogenomics comes in. Pharmacogenomic testing identifies genetic variations that may impact a patient’s response to a medication or therapy. Genetic changes may alter the body’s production of drug-metabolizing enzymes, transporters, targets, and receptors.
The United States Food and Drug Administration (FDA) currently monitors pharmacogenomic information on drug labels for hundreds of drugs. Providers can use this information alongside pharmacogenomic testing to develop a personalized treatment plan with the most ideal drugs and improve clinical outcomes.
Below is a graphical abstract of a 2019 article in Current Applications of Pharmaceutical Biotechnology that outlines the impacts of pharmacogenomics on personalized medicine.
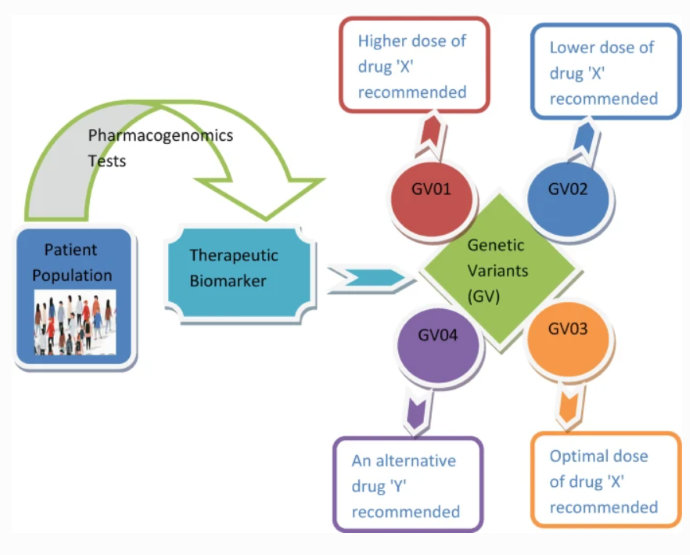
Drug Efficacy
Clinical implementation of pharmacogenomic data can be used to optimize drug efficacy. From choosing the appropriate treatment to managing the dose, optimized drug regimens can improve patient outcomes and reduce the risk of adverse drug events (ADEs).
The National Human Genome Research Institute (NGRI), a subset of the National Institutes of Health (NIH), notes that pharmacogenomic data is only routinely considered in particular specialties, such as cancer care. However, experts predict that pharmacogenomic data advances could be applied beyond oncology to prescribe medications for other conditions across multiple care settings, such as heart disease, asthma, and depression.
The institute notes that some providers may order pharmacogenetic testing before prescribing a treatment plan for patients with breast cancer. The link between genetic mutations and some types of breast cancer is well-established, meaning that healthcare professionals can use genetic data to inform clinical decision-making.
More specifically, trastuzumab is a chemotherapy for patients with genetic variability in the HER2 genes, resulting in overproduction of the corresponding protein. Breast cancer patients without this variation will not benefit from this drug. Instead of a trial-and-error approach, clinicians can optimize the treatment plan to exclude or include this drug depending on the patient’s genetic profile.
In addition, ThermoFisher Scientific explains that clinical pharmacogenomics in psychiatric care can improve patient outcomes. The organization estimates that PGx-guided drug therapy for patients undergoing mental health treatment results in 40% fewer emergency room visits and nearly 60% fewer hospitalizations.
Drug Safety
Beyond improving efficacy, understanding a patient’s genetic information and how it impacts medication metabolism and processing can facilitate safer treatments and reduce the risk of ADEs.
“Genetic makeup-based prescription, design, and implementation of therapy not only improve the outcome of treatments but also reduce the risk of toxicity and other adverse effects,” noted researchers in Current Applications of Pharmaceutical Biotechnology.
Multiple applications of PGx can improve patient safety when beginning a new medication or treatment regimen. For example, patients with human immunodeficiency virus (HIV) who start or alter their antiretroviral therapy (ART) may undergo pharmacogenomic testing. According to the NGRI, specific genetic variants can increase the risk of adverse reactions to abacavir. Pre-emptive pharmacogenomic testing can help prescribers and patients avoid the risks associated with adverse events.
Beyond clinical decision support for patients with HIV, applying pharmacogenomics sequencing in cancer care can minimize the incredibly toxic effects of some chemotherapy drugs. The FDA recommends genetic testing for patients with acute lymphoblastic leukemia (ALL) whose provider may be considering prescribing mercaptopurine. Some genetic variations can impact a patient’s ability to process the drug, causing adverse drug reactions. Similarly, the FDA recommends pharmacogenomic testing before prescribing irinotecan.
In addition to avoiding adverse drug–gene interactions, PGx can be a critical safety tool in polypharmacy for understanding drug–drug interactions ThermoFisher explained that PGx can be used to understand side effects and interactions for patients taking multiple drugs for various conditions or patients on a multi-drug regimen.
For home health patients, PGx is associated with 52% fewer rehospitalizations, 42% fewer emergency room visits, and an 85% reduction in polypharmacy-associated mortality.
Overall Treatment Costs
According to the University of Pittsburgh Institute for Precision Medicine, medication errors and adverse side effects cost the United States healthcare system approximately $40 billion annually.
By optimizing treatment approaches, PGx has the potential to reduce overall treatment costs and healthcare spending significantly. Actionable data from PGx can guide providers toward effective treatment early in the therapeutic process better than a trial-and-error approach. The healthcare system may substantially reduce the costs associated with ineffective treatment or adverse reactions by conducting pharmacogenomic testing.
Current Applications of Pharmaceutical Biotechnology notes, “A better understanding of individual variations and their effect on drug response, metabolism excretion, and toxicity will replace the trial-and-error approach of treatment.”
First, pharmacogenomic testing may shorten the treatment pathway, allowing providers to get to the most effective treatment without a trial-and-error process. For example, in psychiatry, clinicians currently prescribe selective serotonin reuptake inhibitors (SSRIs) for patients dealing with depression. However, these medications can take an extended period to work. Additionally, patients often go through multiple antidepressants before finding an effective one.
The implementation of pharmacogenomics in clinical practice to understand how genetics may impact the efficacy of a drug can point healthcare providers in the right direction, cutting down treatment time, cost, and burdens. For example, a study published in JAMA Network revealed that pharmacogenomic-guided treatment for major depressive disorder (MDD) increased the probability of remission by 28% at 24 weeks.
A systematic review published in Clinical Pharmacology and Therapeutics revealed that — across 108 studies that evaluated 38 drugs — 71% revealed that PGx testing is cost-effective or cost-saving, indicating a financial benefit to PGx testing.
Research and Development
Besides direct patient care and clinical utility, PGx can also advance drug discovery, research, and development.
According to a 2018 article published in the International Journal of Biomedical Investigation, genomic data and computational models can double a researcher’s probability of identifying an appropriate drug target.
“Until recently, drug developers usually used an approach that involved screening for chemicals with broad action against disease. Researchers are now using genomic information to find or design drugs aimed at subgroups of patients with specific genetic profiles. In addition, researchers are using pharmacogenomic tools to search for drugs that target specific molecular and cellular pathways involved in disease,” notes the NGRI.
Researchers in the article also recommend that investigators incorporate pharmacogenomic testing in phase 2 clinical trials to facilitate better data analysis and understanding of medication efficacy.
Despite the extensive perceived benefits of PGx, CVS Health warns that not all pharmacogenomic tests are clinically validated. Unlike well-researched, evidence-based tests, unvalidated pharmacogenomic testing cannot confidently be used to inform prescribing.
Researchers should continue to develop, assess, and validate pharmacogenomic testing to enhance precision medicine and improve healthcare outcomes.






