
Getty Images
Policy in Practice: How the Influenza Act Would Influence Healthcare
If approved, the Influenza Act, introduced in December 2022, could influence healthcare practices surrounding seasonal and pandemic influenza.
On December 8, 2022, Democratic Representative Rick Larsen introduced the Protecting America from Seasonal and Pandemic Influenza Act of 2022 — referred to as the Influenza Act. Backed by other representatives, clinicians, and public health organizations, this bill, if passed, will allocate federal resources to addressing seasonal and pandemic flu concerns, influencing multiple aspects of healthcare.
“The Influenza Act, to our knowledge, is the first attempt to enact a comprehensive authorizing bill with policy changes around seasonal and pandemic influenza,” explained Niki Carelli, executive director of the Coalition to Stop Flu.
As of January 11, 2023, the United States has already had 74 influenza-related deaths this year, 13 among pediatric populations. The CDC’s FluView Data notes that this season’s cumulative hospitalizations rate is 48.6 per 100,000 people. With an early start to this season’s flu cases, the US faces some of the highest influenza infection rates in recent years.
The elevated rates of infection and disease spread have impacted nearly all communities across the US. Straining the already faulty pharmaceutical supply chain, increased influenza infections — and rising rates of respiratory infections, such as RSV and COVID — have led to shortages in over-the-counter cold or flu medications and prescription antivirals, like Tamiflu. Although infection rates have begun to decline, these challenges have emphasized the need for additional influenza prevention and treatment efforts by public and private organizations.
Current Influenza Policies and Federal Involvement
Carelli comments that “the legislative landscape around flu and how the federal government approaches both seasonal and pandemic flu has been static, which is very troubling. We as a country seem to have almost accepted as a status quo that there will be tens of thousands of deaths annually from seasonal flu.”
She notes that multiple different federal agencies play a role in flu policy. The graphic below, provided by the coalition, separates federal involvement in flu and influenza policy into five distinct categories:
- outbreak prevention
- threat identification and early warnings
- infection detection and diagnosis
- vaccine and treatment development or protection
- disease response and recovery to minimize the spread
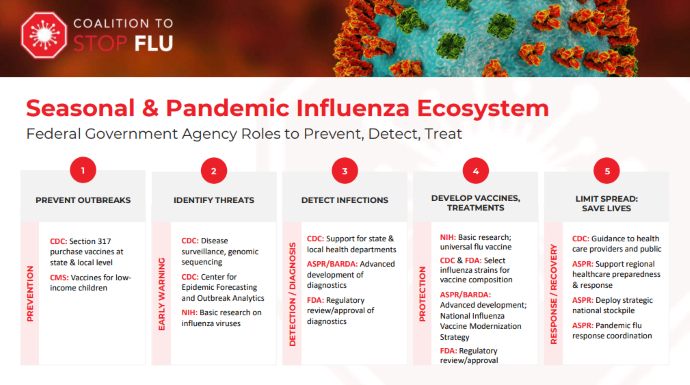
Many infectious diseases, like COVID, follow this same pattern of government involvement in enhancing public health. While many policies and organizations overlap in their efforts to address these five factors, each plays a significant role. According to Carelli, the CDC and ASPR play the most prominent role in the legislative landscape around influenza.
The Influenza Act
Despite the multitude of policies, many believe that current regulations and public health efforts must be revised. The Influenza Act attempts to strengthen and enhance the current policies around pandemics and seasonal influenza.
“This bill takes a holistic look at all the changes the federal government could make to improve that effort,” noted Carelli. “It addresses strengthening and diversifying influenza vaccine development, manufacturing, and supply chains, promoting innovative approaches and use of new technologies to detect, prevent, and respond to flu, increasing the flu vaccine and therapeutics access, and authorizing sustainable funding for the flu ecosystem.”
This multi-part bill addresses the development and delivery of technology, medication, and education surrounding flu. Carelli notes that having the best vaccines and therapeutics in the world without a way to ensure that everyone has access to and utilizes them is just as infective as not having the resources to begin with.
Key Components
The bill has many vital components and actionable requests that promote innovation and equitable, timely access to care. The Influenza Act is divided into four distinct goals or subsections, listed below:
- developing strong, diverse influenza vaccines, manufacturing practices, and supply chains
- promoting the innovative use of new technologies for flu detection, prevention, and response
- increasing vaccine access and coverage
- authorizing sustainable funding for influenza
Key Players
To effectively execute the goals of this bill — including developing treatments and delivering care — federal and private organizations need to collaborate. “Continuing to strengthen public–private partnerships is important. The federal government has built many new partnerships through the work with COVID over the last couple of years, and it's crucial to make sure that those new partnerships and the lessons learned from COVID are translated over to flu and other infectious diseases,” Carelli stated.
“The CDC and ASPR both play significant roles, and agencies like the NIH, FDA, and CMS also have critical roles to play here, too,” she said. “But a lot of the work happens on the ground. So, the state, territorial, and local health officials and providers on the front lines also play a huge role.”
In addition to the public sector, she highlights the importance of the private sector and academic institutions in conducting research and developing treatments or vaccinations for influenza. Beyond development, these private and educational organizations should have plans to distribute influenza treatments and tools.
“Many people call it the flu ecosystem — it's a big ecosystem. Our coalition represents players all across that ecosystem. And so we believe that the bill does a good job at addressing the different elements of that comprehensive effort necessary to deal with seasonal flu and the threat of pandemic flu,” noted Carelli.
Strengthening Vaccine Development, Manufacturing, and Supply Chains
Under the goal of strengthening vaccine development, manufacturing, and supply chains, this act sets seven subgoals with specific plans on how to reach them.
Among these seven sub-goals is the timely delivery of vaccines, universal vaccine development, strengthening the influenza vaccine supply chain, implementing national influenza vaccine modernization strategies, involvement of the assistant secretary for preparedness and response, involving the Biomedical Advanced Research and Development Authority, and implementing a pandemic influenza medical countermeasures program.
These many subgoals require funding the development of vaccines, strengthening public and private partnerships, and collaboration across multiple sectors of healthcare and healthcare policy, building on pre-existing strategies from COVID-19.
“We need to take some of the learnings from COVID. There's been fantastic work done on COVID and COVID vaccination,” stated Carelli. “A lot of us in the infectious diseases community want to ensure that the lessons that we have learned, the funding that has been spent, and the new systems that have been stood up are all looked at for applicability to flu and other infectious diseases too.”
Promoting Innovation
Within the goal of promoting innovation, the act lists plans to expand genomic sequencing, prioritize influenza and pathogen agnostic tools, develop point-of-person diagnostics, enhance diagnostic supply chain resiliency, modernize assays, and implement reporting strategies.
Increasing Coverage
A primary goal of this act is to provide equitable and reliable access to influenza prevention, diagnostic, and treatment tools to all patient populations. As a part of this goal, authors of the bill hope to implement annual reporting on public communication strategies, incorporate health equity into influenza planning and response, report on and extrapolate lessons learned from COVID-19, and monitor the distribution of resources.
“The process of ensuring that the United States public health system has the infrastructure necessary to respond to flu and having effective messaging and programs in place to reach underserved or hesitant populations are incredibly critical,” emphasized Carelli. “Unfortunately, there's not a silver bullet, but we know that much more can be done than we currently do.”
She emphasized that the first part of reaching diverse communities across the country is setting a goal and making a point to include community outreach in legislation.
“That's what the statute does — it puts in law that there needs to be a focus by the administration, to work toward health equity and increase access to vaccines and therapeutics,” added Carelli. “The federal government has a bad habit of siloing. Many people, including myself, want to make sure that all that great work and the things we have learned from COVID are translated over to flu and other infectious diseases so that that effort that we've all spent over the last couple of years is not lost.”
Funding
The final goal of this bill is to authorize sustainable funding for influenza preparedness and response. The act requests that money be allocated from many programs, including the following:
- Influenza Planning and Response Program
- Strategic National Stockpile
- Hospital Preparedness Program
- Immunization Program
- Public Health Emergency Preparedness Program
- Infectious Disease and Rapid Response Reserve Fund
- Data Modernization Initiative
- Universal Flu Vaccine Research








