Getty Images
How Monoclonal Antibodies Are Shaping the Future of Healthcare
Monoclonal antibodies have unleashed the power of precision medicine for various medical conditions.
Monoclonal antibodies (mAbs), a groundbreaking class of therapeutic agents, have emerged as a transformative tool in modern medicine. With their remarkable precision and versatility, these engineered antibodies offer promising solutions across various medical conditions. From cancer treatment to infectious diseases to autoimmune disorders, mAbs are revolutionizing patient care and shaping the future of healthcare.
What Are Monoclonal Antibodies?
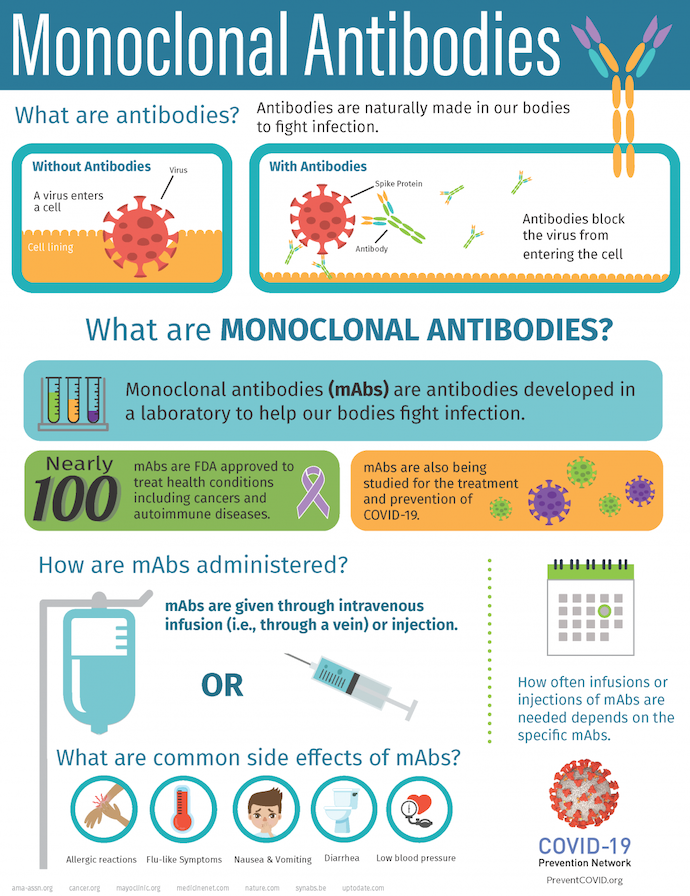
Monoclonal antibodies are laboratory-produced molecules that mimic the body's natural immune response. They are created by cloning a single type of immune cell, a plasma cell, to produce large quantities of identical antibodies. These antibodies can then be specifically targeted to recognize and bind to particular proteins, cells, or pathogens within the body, enabling precise and tailored interventions.
All antibody molecules within mAbs possess identical antigen recognition sites, affinity, biological interactions, and subsequent effects. This sets mAbs apart from polyclonal antibodies, as the latter consists of diverse protein sequences and recognizes varied epitopes.
Monoclonal antibody treatments are primarily administered through intravenous (IV) solutions, commonly in an infusion center.
Monoclonal antibodies are used for diagnosis, disease treatment, and research. They are used as probes to identify materials in laboratories or home-testing kits like those for pregnancy or ovulation, to type tissue and blood for use in transplants, and for diagnosis and disease treatment.
Monoclonal antibodies have found other therapeutic applications in various conditions, including the following:
- Cancer
- Prevention of organ transplant rejection
- Treatment of inflammatory and autoimmune disorders, including allergies
- Management of infections, including COVID-19
- Osteoporosis
- Eye conditions
- Migraines
- High cholesterol
- Disorders affecting the nervous system
Since the approval of the first mAb drug for humans in 1986, the FDA’s approvals for mAb therapies have steadily increased.
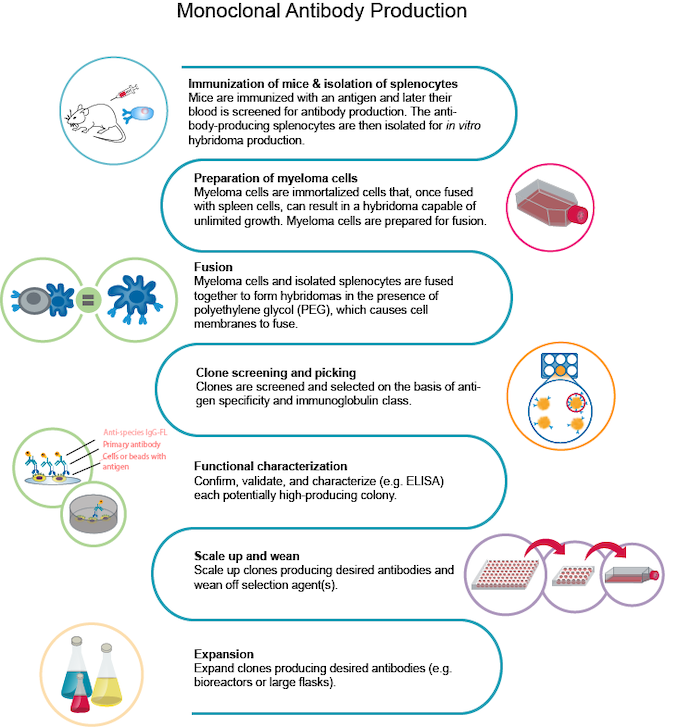
How Are Monoclonal Antibodies Made?
The typical process for producing mAbs involves several steps:
- Immunization of mice and isolation of splenocytes: Mice are immunized with a specific antigen, and their blood is later tested to identify the production of antibodies. Splenocytes, antibody-producing cells, are isolated from the spleen for further processing.
- Preparation of myeloma cells: Myeloma cells, immortalized cells capable of unlimited growth, are prepared for fusion with the splenocytes. These cells will form hybridomas.
- Fusion: The myeloma cells and isolated splenocytes are fused using polyethylene glycol (PEG), which causes the fusion of their cell membranes.
- Clone screening and picking: The resulting hybridomas are screened and selected based on their antigen specificity and immunoglobulin class.
- Functional characterization: Each potentially high-producing colony is further validated and characterized, often using enzyme-linked immunosorbent assay (ELISA) techniques to confirm its functionality.
- Scale up and wean: The selected clones producing the desired antibodies are scaled up in production and gradually weaned off any selection agents used during the process.
- Expansion: The chosen antibody-producing clones are expanded further using bioreactors or large flasks to generate a larger quantity of the desired antibodies.
Forms of Monoclonal Antibodies
Monoclonal antibodies can be employed in different forms:
- Administered as a standalone therapy, referred to as "naked" mAbs
- Converted into radioactive particles and combined with another drug, serving as therapy — also called "conjugated," "tagged," "loaded," or "labeled" mAbs
- Modified to target and attack two specific antigens simultaneously, known as "bispecific" mAbs
Monoclonal antibodies are synthetic proteins that can be produced in four different ways, each named based on their composition:
- Murine: These antibodies are derived from mouse proteins, and the names of treatments using them typically end in "-omab."
- Chimeric: These antibodies are a combination of mouse and human proteins, with the names of related treatments ending in "-ximab."
- Humanized: These antibodies consist of small portions of mouse proteins fused with human proteins, and treatments using them are named with the suffix "-zumab."
- Human: These antibodies are entirely composed of human proteins, and the names of treatments utilizing them end in "-umab."
What Are Conjugated Monoclonal Antibodies?
Conjugated mAbs involve the combination of mAbs with a chemotherapy drug or a radioactive particle. These conjugated mAbs function as targeting agents, guiding the chemotherapy drug or radioactive substance directly to cancer cells. The mAb circulates within the body until it finds and attaches to the specific target antigen, delivering the toxic substance precisely where needed. This approach minimizes damage to normal cells in other areas of the body. Conjugated mAbs may also be called tagged, labeled, or loaded antibodies.
There are two types of conjugated mAbs:
- Radiolabeled antibodies: These antibodies are equipped with small radioactive particles. Ibritumomab tiuxetan (Zevalin) is an example of a radiolabeled mAb. It specifically targets the CD20 antigen present on B cells. By delivering radioactivity directly to cancer cells, this antibody combines the mAb drug (rituximab) with a radioactive substance (Yttrium-90). Treatment with this type of antibody is sometimes called radioimmunotherapy (RIT), as it allows the drug and radiation to be directed precisely to the target cells, affecting the target and nearby cells to a certain extent.
- Antibody—drug conjugates (chemolabeled antibodies): These mAbs are linked with potent chemotherapy drugs or other therapeutic agents. Examples include Brentuximab vedotin (Adcetris), which targets the CD30 antigen found on lymphocytes, combined with a chemotherapy drug called MMAE. Ado-trastuzumab emtansine (Kadcyla or TDM-1) is another example, targeting the HER2 protein and linked to a chemotherapy drug known as DM1.
What Are Bispecific Monoclonal Antibodies?
These medications consist of segments derived from two distinct mAbs, allowing them to bind to two different proteins simultaneously. A prime example is blinatumomab (Blincyto), used to treat certain leukemia forms. One component of blinatumomab connects to the CD19 protein present on specific leukemia and lymphoma cells. In contrast, the other component attaches to CD3, a protein present on T cells, a type of immune cell. By binding to CD19 and CD3, this drug facilitates the interaction between cancer cells and immune cells, leading to an immune system response against the cancer cells.
FDA-Approved Monoclonal Antibodies
The FDA has approved over 100 mAbs, making them prominent in the pharmaceutical landscape. According to a 2021 Nature Reviews Drug Discovery article, these mAbs constitute 9 out of the top 20 therapeutic products worldwide in terms of sales, accumulating revenue surpassing $75 billion. Projections indicate that the global market for therapeutic mAbs will experience substantial growth, reaching over $300 billion by 2025, primarily fueled by the continuous development of novel mAbs, as noted in the Journal of Biomedical Science.
Monoclonal Antibodies in Oncology
One of the most significant applications of mAbs is in oncology. These antibodies have revolutionized cancer treatment by directly targeting cancer cells or blocking the signals that promote their growth. Examples include trastuzumab, used to treat HER2-positive breast cancer, and rituximab, used for certain types of lymphoma and leukemia. mAbs have demonstrated remarkable efficacy in improving patient outcomes, reducing side effects, and increasing survival rates.
Monoclonal Antibodies for Infectious Diseases
Infectious diseases also present a fertile ground for the use of mAbs. They can neutralize pathogens, prevent viral entry into host cells, or modulate the immune response. Monoclonal antibodies, such as palivizumab, have been successfully utilized to prevent respiratory syncytial virus (RSV) infections in high-risk infants. In the context of emerging infectious diseases, mAbs have shown promise in treating conditions like COVID-19, where they can target the spike protein of the SARS-CoV-2 virus and inhibit its ability to infect human cells.
Monoclonal Antibodies for Autoimmune Disorders
Autoimmune disorders, characterized by an overactive immune response against the body's tissues, can also benefit from mAb therapy. Antibodies engineered to block specific molecules involved in the disease process can help alleviate symptoms and slow disease progression. Examples include adalimumab and infliximab, which target tumor necrosis factor (TNF) and are used to treat conditions like rheumatoid arthritis, Crohn's disease, and psoriasis. Monoclonal antibodies have revolutionized the management of these chronic conditions, improving the quality of life for countless patients.
Expanded Applications
Monoclonal antibodies are not limited to cancer, infectious diseases, and autoimmune disorders. They are increasingly being explored for use in cardiovascular diseases, neurological disorders, and even as targeted drug delivery systems. Their adaptability and potential fuel ongoing research and innovation, with new therapeutic applications continuously being discovered.
Using mAbs as a preventive measure against malaria is being explored as an alternative to vaccines. Research has shown that administering antibodies directly to individuals can effectively protect against malaria infection.
In a trial conducted in Mali with 300 participants, a laboratory-made antibody demonstrated up to 88% protection against infection over six months. The study involved intravenous antibody delivery, although subcutaneous or intramuscular injections could be used for young children.
Challenges, Costs, and Risks
Despite their immense potential, it is crucial to address challenges related to cost, accessibility, and safety. Monoclonal antibody therapies can be expensive, limiting access for some patients.
Manufacturing a mAb can be intricate and costly, with an average price ranging from $6,000 to $15,000. In contrast, polyclonal antibodies are less expensive, and their production cost can be around $1,000 when utilizing a straightforward small molecule antigen.
Infusion reactions, occurring during or after mAb treatment, involve a robust immune response. Common signs include the following:
- Weakness
- Headache
- Nausea
- Vomiting
- Diarrhea
- Low blood pressure
- Rashes
- Fever
These reactions are more likely during the initial administration of the drug due to the protein-based nature of mAbs.
Compared to chemotherapy drugs, naked mAbs generally have fewer severe side effects. However, they can still cause issues in certain individuals. Some mAbs may lead to side effects associated with the antigens they target:
- Bevacizumab (Avastin) is an mAb that targets VEGF, a protein involved in tumor blood vessel growth. It can potentially cause side effects like high blood pressure, bleeding, delayed wound healing, blood clots, and kidney damage.
- Cetuximab (Erbitux) is an antibody that targets EGFR, a cell protein found in both normal skin cells and specific cancer cells. This drug can result in severe rashes for some people.
Managing these reactions can involve slowing the infusion rate or reducing the dosage to mitigate their impact.
While less frequent, more severe risks are associated with undesired immune system reactions, such as acute anaphylaxis, cytokine release syndrome (CRS), and serum sickness.
Acute anaphylaxis is an intense allergic reaction that can be life-threatening. Serum sickness occurs when the immune system attacks the antiserum or blood product containing the proteins utilized in your treatment. CRS, also known as cytokine storm, has the potential to cause organ damage.
It's important to note that some risks related to mAb therapy are specific to the particular condition being treated. For instance, tumor lysis syndrome, typically associated with cancer treatment, can lead to kidney failure.
Collaboration and Future Prospects
As mAb therapy continues to evolve, collaboration between researchers, clinicians, pharmaceutical companies, and regulatory agencies remains essential. Together, they can advance the understanding of these powerful agents, accelerate the development of new therapies, and ensure their safe and effective integration into medical practice.
The rapid progress in mAb technology heralds a new era in medicine, offering hope to patients across diverse medical conditions. With their remarkable specificity and therapeutic potential, these innovative agents are rewriting the rules of medical treatment, providing personalized and targeted interventions that were once unimaginable. As research and clinical experience expand, mAbs are poised to shape the future of healthcare, ushering in an era of precision medicine and improved patient outcomes.