
filo/DigitalVision Vectors via G
FDA-approved insulin pumps used for insulin pump therapy
FDA-approved insulin pumps offer precise insulin pump therapy, revolutionizing diabetes management with personalized care.
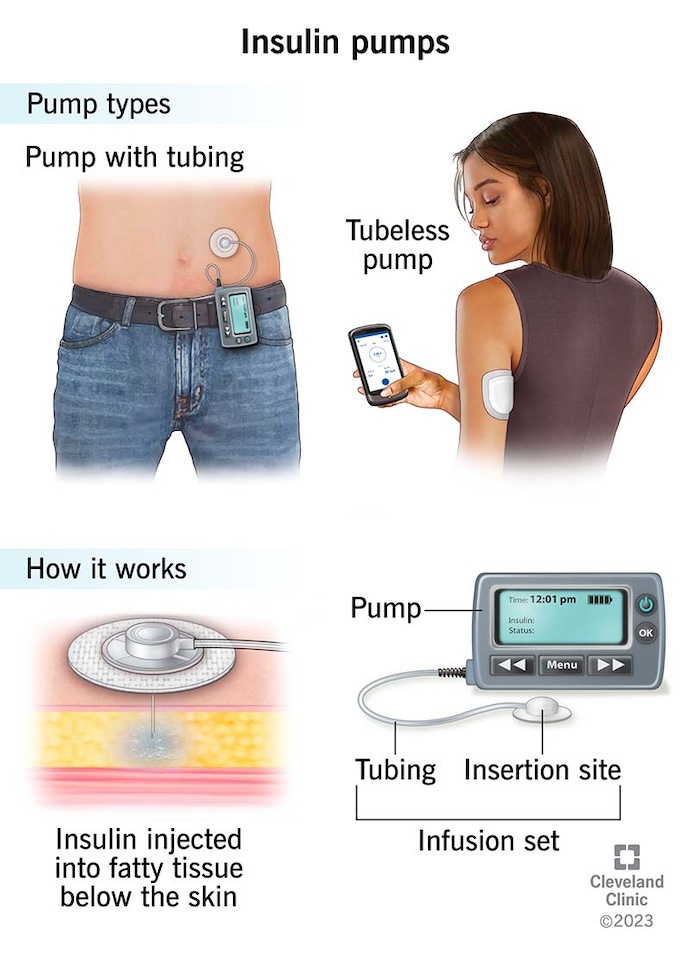
Insulin pump therapy, also known as continuous subcutaneous insulin infusion (CSII), has emerged as a significant advancement in diabetes technology over the last 50 years. Although commercial insulin pumps were introduced in the 1970s, widespread adoption didn't occur until the early 2000s, following the Diabetes Control and Complications Trial (DCCT) in the early 1990s. The DCCT emphasized the importance of intensive insulin therapy for meticulous glycemic control and preventing diabetes-related complications.
Since then, insulin pump technology has rapidly evolved to closely mimic physiological insulin secretion, aiding patients in achieving precise glycemic control while minimizing hypoglycemia risk.
Insulin Pump Devices
An insulin pump is a compact, wearable device that administers short-acting insulin in small increments every few minutes throughout the day and night. It can also provide additional doses of short-acting insulin with the press of a button, either during meals or to lower high blood glucose levels. Insulin pumps generally come in two types: traditional tubed and tubeless (Figure 1).
The adoption of insulin pumps has grown significantly, with over 350,000 users in the US today, primarily those with type 1 diabetes (T1D). The insulin pump market is projected to reach 10.3 billion USD by 2032, driven by a compound annual growth rate of 8.1% from 2023 to 2032, according to DataHorizzon Research.
FDA-Approved Insulin Pump Devices
In the US, insulin pump systems that have passed FDA clearance include the following devices:
- Tandem’s t:slim X2 Insulin Pump with Basal-IQ; approved on June 21, 2018
- Insulet’s Omnipod DASH Insulin Management System; approved on September 20, 2019
- Insulet's Omnipod 5 Automated Insulin Delivery System; approved on January 22, 2022
- Medtronic’s MiniMed 780G System; approved on April 21, 2023
- Insulet's Omnipod GO; approved on April 25, 2023
- Beta Bionics’ iLet ACE Pump/iLet Dosing Decision Software; approved on May 19, 2023
- Roche’s Accu-Chek Solo Micropump System; approved on August 10, 2023
- Sequel’s twiist Automated Insulin Delivery System; approved on March 18, 2024
Tandem Diabetes Care’s t:slim X2
Approved in June 2018, the t:slim X2 insulin pump with Basal-IQ Technology from Tandem Diabetes Care is an advanced device designed to assist individuals with diabetes in managing their insulin therapy. It features an easy-to-use color touchscreen interface, like a smartphone, allowing users to navigate various menus and settings. Through wireless connectivity, the pump can be connected to compatible devices, such as automated insulin dosing software or continuous glucose monitoring (CGM) systems.
When used with the Dexcom G5 Mobile CGM or compatible iCGMs, the Basal-IQ Technology can suspend insulin delivery based on CGM sensor readings. This device utilizes predictive technologies to automate insulin delivery based on real-time glucose data, helping users maintain optimal glucose levels. The pump holds a reservoir or cartridge filled with insulin, delivered subcutaneously through a small cannula or tubing. Overall, the t:slim X2 insulin pump offers a user-friendly interface, wireless connectivity, and predictive technologies to enhance insulin therapy management for individuals with diabetes.
Key features include the following:
- Tubed
- Easy-to-use color touchscreen interface
- Wireless connectivity for communication with compatible devices
- Compatibility with automated insulin dosing software and CGM systems
- Predictive technologies for automated insulin delivery
- Customizable insulin delivery settings for individual needs
- Compact and portable design for convenient use on-the-go
Insulet's Omnipod DASH Insulin Management System
Approved in September 2019, Insulet’s Omnipod DASH Insulin Management System, developed by Insulet Corporation, caters specifically to individuals managing T1D. Unlike traditional insulin pumps, it offers a tubeless design, enhancing flexibility and ease of use for insulin therapy recipients.
Key features of the Omnipod DASH system include the following:
- Tubeless
- Small, waterproof pod houses insulin reservoir, infusion set, and insertion mechanism
- Wireless communication allows remote insulin delivery control and glucose monitoring
- User-friendly interface on PDM enables intuitive navigation
- Basal rates, bolus doses, and settings are easily tailored to individual needs
- Tools for tracking insulin delivery and glucose levels aid decision-making
- Auto-off feature suspends insulin delivery after inactivity
- Disposable pods eliminate the need for refilling or changing components
Additionally, the disposable pod eliminates the need to refill insulin cartridges or change infusion sets but adds to the mounting issue of medical waste.
Insulet's Omnipod 5
In January 2022, the Omnipod 5 automated insulin delivery (AID) system from Insulet gained FDA approval for individuals aged 6 and above with T1D, with a limited release, making it the initial tubeless AID system for individuals with T1D requiring both basal and mealtime insulin. This AID system was released to the market on August 1, 2022, and gained expanded approval on August 22, 2022, to include individuals aged 2 and above with T1D.
The Omnipod 5 is a fully integrated closed-loop automated insulin delivery (AID) system. It combines a wearable insulin delivery pod with a smartphone app, eliminating the need for a separate controller device. The Omnipod 5 also features SmartAdjust technology, activity features, a SmartBolus calculator, and a waterproof construction. It also offers advanced features and integration with the Dexcom G6 continuous glucose monitoring (CGM) system.
Key features include the following:
- Tubeless design for freedom of movement and discretion
- Easy application and removal process
- Long-acting insulin delivery for basal insulin needs
- FDA-approved for individuals with T2D aged 18 or older
Medtronic MiniMed 780G System
Approved in April 2023, The Medtronic MiniMed 780G System is an automated insulin delivery system that helps individuals aged 7 and older manage their diabetes. This system continuously monitors glucose levels, delivers insulin automatically, and adjusts the dosage as needed. It provides real-time glucose trend information, alerts, and alarms, allowing users to make informed decisions about their diabetes management.
Key features include the following:
- Tubed
- CGM for accurate and timely glucose level readings
- Bluetooth connectivity for wireless transmission of glucose data to the insulin pump
- Advanced hybrid closed-loop algorithm for personalized insulin delivery
- FDA-approved for individuals seven years of age and older with T1D
Insulet's Omnipod GO
Insulet's newest AID system, Omnipod GO, received FDA clearance in April 2023 and is currently commercializing its long-acting insulin delivery device. Designed for individuals with type 2 diabetes (T2D) aged 18 or older, Omnipod GO covers the basal-only insulin population. This device offers a discreet and tubeless design, eliminating the need for traditional insulin pump tubing. Users can easily apply and remove the device, providing convenience and flexibility in their daily activities. Omnipod GO is a standalone device that does not connect to any devices.
The Omnipod GO is a simplified version of the Omnipod system, comprising a wearable insulin delivery pod without the integrated closed-loop system or smartphone app. The Omnipod GO is designed to provide a more affordable and accessible option for individuals who may not require the advanced features of the Omnipod 5.
Key features include the following:
- Tubeless and waterproof
- Standalone and wearable
- Delivers a fixed rate of non-stop rapid-acting insulin over a three-day period
- Offers seven pre-programmed daily rates, ranging from 10 to 40 units per day
- Operable without a separate handheld device
- Compatible with various U-100 insulins
Beta Bionics’ iLet ACE Pump and iLet Dosing Decision Software
The Beta Bionics iLet ACE Pump and iLet Dosing Decision Software received FDA clearance in May 2023. This system, along with a compatible integrated continuous glucose monitor (iCGM), forms the iLet Bionic Pancreas. The iLet Bionic Pancreas is an automated insulin dosing (AID) system that utilizes an algorithm to determine and command insulin delivery.
Key features include the following:
- Tubed with adaptive closed-loop algorithm for personalized insulin dosing
- Simplified mealtime insulin dosing with a meal announcement feature
- No need for manual adjustment of insulin pump therapy settings
- FDA-approved for individuals six years of age and older with T1D
Roche’s Accu-Chek Solo Micropump System
Approved in August 2023, the Roche Accu-Chek Solo Micropump System, developed by Roche Diabetes Care, offers individuals with diabetes a convenient and flexible way to manage their insulin therapy. The Accu-Chek Solo Micropump System is a tubeless patch pump that integrates the insulin pump, infusion set, and reservoir into a single device, simplifying the entire system.
The system delivers insulin subcutaneously through a small cannula inserted into the skin and allows for customizable basal rates and bolus doses to meet individual insulin needs. The Accu-Chek Solo Micropump System includes user-friendly features such as an integrated and illuminated Accu-Chek Guide test strip port for blood glucose monitoring. It also features a clinically proven bolus advisor to calculate insulin doses. Users can set up to five different basal rate profiles, providing flexibility in insulin delivery based on daily needs. Additionally, the system offers an MDI mode, allowing users to temporarily switch to using a pen for insulin delivery without losing important data.
Key features included the following:
- Tubeless
- Compact and lightweight for minimal awareness and effortless concealment.
- Adapts to patients' preferences
- Conveniently adheres to various infusion sites and is detachable as needed
- Administer bolus insulin with or without the diabetes manager handset
- Replace only disposables, preserving the pump unit
Sequel Med Tech’s Twiist Automated Insulin Delivery System
In collaboration with DEKA R&D, Sequel Med Tech for the twiist automated insulin delivery (AID) system powered by Tidepool in March 2024. This system, the first of its kind, directly measures insulin volume and flow with each micro-dose, offering tailored dosing for T1D patients aged six and above. Combining data from CGM devices, control algorithms, and insulin pumps, the twiist AID system automates insulin delivery while leveraging FDA interoperability standards for personalized treatment. Its Tidepool Loop technology adjusts insulin delivery automatically based on CGM readings and predicted glucose levels.
- Tubed
- Provides automatic insulin adjustments based on CGM readings and predicted glucose levels
- Direct measurement of insulin volume and flow accompanying every micro-dose
- Cleared for individuals aged 6 and older with T1D
- Offers personalized dosing and flexibility to address each patient's needs
- Simplifies daily life for T1D patients
- Expected to be available through pharmacies for convenient access
FDA-approved insulin pump devices have transformed the landscape of diabetes management, offering individuals with diabetes greater control, convenience, and flexibility. These devices provide personalized insulin delivery, improved glucose control, and enhanced quality of life for individuals with diabetes.




