
sdecoret - stock.adobe.com
Exploring the 5 FDA-Approved IUDs: Types, Benefits, and Effectiveness
IUDs are a primary tool to control reproduction, benefit public health, empower individuals, and enhance healthcare outcomes.
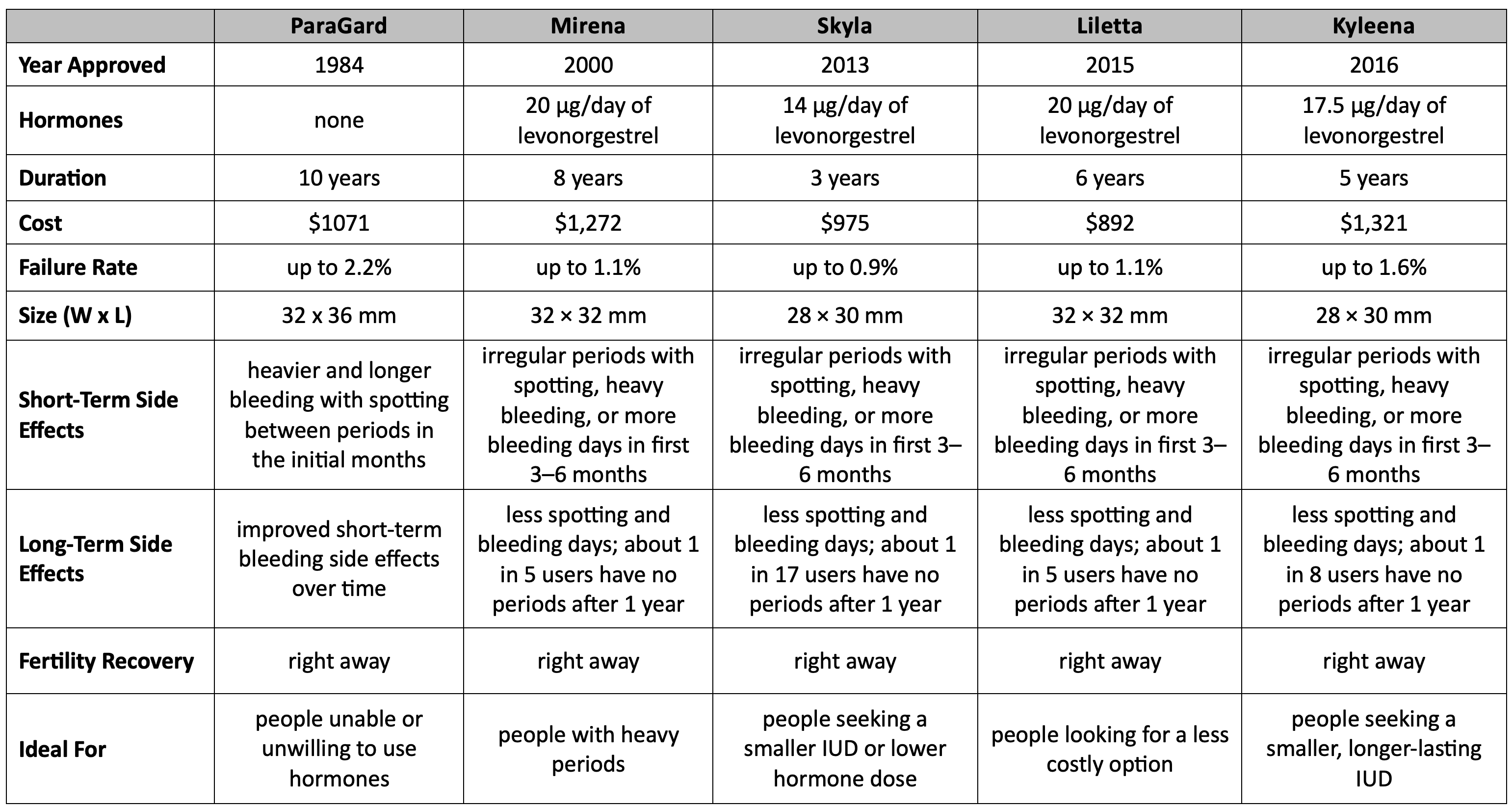
Intrauterine devices (IUDs) are a form of long-acting reversible contraception (LARC) inserted into the uterus to prevent pregnancy. They are small, T-shaped devices made of either copper or plastic with a hormone-releasing component. IUDs work by altering the cervical mucus, inhibiting sperm movement, and affecting the uterine lining to prevent fertilization and implantation of a fertilized egg. As of August 2023, there are five FDA-approved IUDs on the United States market.
The national utilization of IUDs has risen since the early 2000s, although it remains lower than other contraceptive methods. The expense can be a potential obstacle for more women to adopt LARC. Fortunately, numerous insurance plans and government programs offer coverage for IUD costs.
There has been a notable shift in attitudes toward the safety and effectiveness of IUDs, generating increased interest, particularly among younger providers and people less familiar with the past controversies surrounding IUDs.
Following birth control pills, IUDs rank second among the most utilized reversible birth control methods. On a global scale, approximately 23% of women and individuals assigned female at birth who use contraception opt for IUDs. In the US, the prevalence of IUD usage surged from 2% to 14% between 2002 and 2014.
According to the Centers for Disease Control and Prevention (CDC), approximately 10.4% of women between 15 and 49 depend on IUDs or implants as their chosen contraceptive method. In comparison, 14% prefer using the pill, while 18.1% rely on female sterilization.
“The ability to prevent pregnancy is very high. The chance of pregnancy with normal use is 1 in 400,” said Hannah Galey, MD, The Christ Hospital, Obstetrics and Gynecology. “In comparison, oral contraceptive pills have about an 8 in 100 chance of pregnancy with normal use. The IUD also has other benefits outside of birth control, including decreased pain with periods and a high rate of not having a period at all (amenorrhea).”
There are two main types of IUDs: copper (nonhormonal) and hormonal.
Copper IUDs
Copper IUDs are hormone-free and primarily work by using the natural properties of copper to create an environment toxic to sperm. While copper IUDs provide longer-term contraception than hormonal IUDs, they have a slightly higher failure rate.
ParaGard
ParaGard, manufactured by Teva Women's Health Pharmaceuticals, received FDA approval in 1984 and has been accessible in the US since 1988. ParaGard is non-hormonal and contains copper, making it the oldest IUD and copper IUD approved by the FDA.
Measuring 32 mm horizontally and 36 mm vertically, with a 3 mm diameter bulb at the tip of the vertical stem, ParaGard provides long-term contraception by preventing sperm from reaching and fertilizing the egg. ParaGard is approved for a maximum of 10 years, but evidence-based use suggests it can be effective for 12–20 years. The average cost of ParaGard is around $1,071 without insurance.
For the first year, the copper IUD demonstrates effectiveness like tubal sterilization. ParaGard’s first-year failure rate ranges from 0.5% to 0.8%; at 7 years, the failure rate ranges from 1.4% to 2.2%. At 12 years, the cumulative failure rate is 2.2%.
Hormonal IUDs
The four FDA-approved hormonal IUDs include Mirena, Skyla, Liletta, and Kyleena, which release a progestin hormone — levonorgestrel — into the uterus. The hormone thickens the cervical mucus, which prevents sperm from reaching the egg, and also thins the uterine lining to make it less receptive to implantation. Hormonal IUDs can provide contraception for various durations, ranging between 3 and 8 years.
Hormonal IUDs offer benefits related to reproductive health aside from contraception. They are often prescribed to individuals with endometriosis, chronic pelvic pain, and heavy periods. Many people also choose hormonal IUDs since they may lessen period pain, reduce period frequency, or eliminate menstruation.
Research suggests that IUDs can be safely and effectively provided to individuals with a uterus who have not given birth, even though they are not officially approved for this group.
Mirena
Mirena, manufactured by Bayer Healthcare Pharmaceuticals, was initially approved by the FDA as a long-acting contraceptive on December 6, 2000. On October 2, 2009, it received additional approval for treating heavy menstrual bleeding among IUD users. The FDA extended its approval for pregnancy prevention up to 7 years on August 25, 2021. Then, on August 18, 2022, the FDA made Mirena the longest-lasting hormonal IUD available in the American market by extending approval for up to 8 years.
With a failure rate of up to 1.1%, Mirena prevents pregnancy by reducing ovulation frequency, thickening cervical mucus, impeding sperm movement into the uterus, and inhibiting sperm from binding to an egg.
Mirena comprises 52 mg of levonorgestrel, initially releasing approximately 20 µg/day. This release rate gradually reduces to half its initial value over 5 years. The cost of Mirena without insurance is roughly $1272.
Skyla
Skyla — approved by the FDA in early 2013 — is one of the two smallest-sized hormonal intrauterine devices. Its 13.5 mg levonorgestrel release is considered a more suitable option for individuals who have never given birth. Its functioning and failure rate (up to 0.9%) resemble Mirena. Due to its slow-release mechanism, only small amounts of levonorgestrel enter the bloodstream, making it a better option for patients sensitive to hormones.
Skyla, manufactured by Bayer, is approved for up to 3 years. The dimensions of its T-body are 28 mm × 30 mm, and the outer diameter of the placement tube is 3.8 mm. Skyla retails for $975.
Liletta
Liletta, approved in 2015, is another hormonal IUD that slowly releases levonorgestrel. It works similarly to Mirena and Skyla but is approved for up to 6 years of use. Liletta has a failure rate of up to 1.1% and retails for around $892.
Developed by Actavis in collaboration with Medicines360, a non-profit women's pharmaceutical company, to provide an affordable IUD option, Liletta is made accessible to public health clinics enrolled in the national 340B Drug Pricing Program, offering significant discounts. The 340B program enables providers serving low-income populations to obtain pharmaceuticals at reduced costs.
Kyleena
Kyleena, the latest to gain FDA approval in September 2016, also slowly releases a low dose of levonorgestrel, providing contraception for up to 5 years. Like Mirena and Skyla, Kyleena is also manufactured by Bayer but distinguishes itself from Mirena by containing lower levels of hormones. Kyleena has a failure rate of up to 1.6% and retails for $1,321.
Table 1. US FDA-Approved IUDs
Insertion—Removal Costs and Side Effects
According to the Student Life University Health Service at the University of Michigan, inserting any of these devices costs $202. In contrast, removing an existing device is priced at $210. If both removal and insertion of a new device are required, the combined cost is $649.
These prices may vary depending on the healthcare provider and insurance coverage. Verifying the current pricing with a patient’s healthcare provider or insurance company is advisable.
For those with health insurance, the Affordable Care Act (Obamacare) ensures that hormonal and non-hormonal IUDs are available for free or at a reduced cost. The Act mandates insurance plans to cover at least one of the 18 FDA-approved birth control methods.
Insurance plans are required to provide coverage for these services without requiring any copayment, coinsurance, or meeting the deductible as long the provider is in-network.
“The risks/side effects of any IUD after placement include cramping, bleeding between your periods, infection in the uterus, a uterine perforation (a hole in the wall of the uterus), unintended pregnancy, and expulsion or rejection of the IUD,” explained Galey. “In addition, the side effects of the copper IUD include a slight increase in bleeding each month. Additional side effects of a progestin IUD include having no period.”
Uterine perforation resulting from the insertion of an intrauterine device is rare, observed in approximately 1 out of every 1,000 insertions. Perforation can manifest as either complete, where the device is entirely within the abdominal cavity, or partial, with the device partially embedded to varying extents within the uterine wall.
Conversely, some studies suggest that IUD use may decrease the risk of certain gynecological cancers, such as ovarian and endometrial. These additional benefits make IUDs a versatile option for reproductive health beyond their primary use as a contraceptive method.






