
Peshkova/istock via Getty Images
Exploring RNA-based therapeutics beyond mRNA vaccines
RNA therapeutics beyond mRNA vaccines can have revolutionary impacts on the healthcare industry, providing new tools to care for patients.
RNA therapeutics have emerged as a critical tool for the healthcare industry. In recent years, mRNA vaccines have been one of the most notable RNA therapeutics, which played a crucial role in curbing the effects of the COVID-19 pandemic. mRNA vaccines, including those manufactured by Pfizer, Moderna, Johnson and Johnson, and Novo Nordisk, are now an indispensable tool in the public health arsenal against COVID-19, providing protection against infections and minimizing the impact for those who contract the virus.
However, the impacts and applications of RNA science and technology reach far beyond vaccines and COVID-19. In this article, LifeSciencesIntelligence will explore the different RNA-based therapeutics, their applications, and the impacts they have had on the healthcare industry.
Below is a timeline of RNA therapeutic discoveries from Nature.
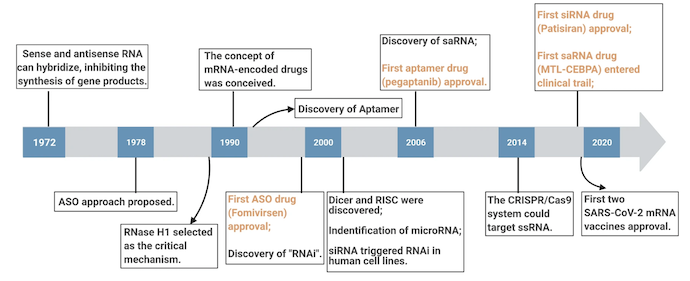
According to the International Journal of Molecular Sciences, there are five primary categories of RNA therapeutics:
- mRNA therapeutics
- Antisense oligonucleotides
- Small interfering RNAs
- MicroRNAs
- Aptamers
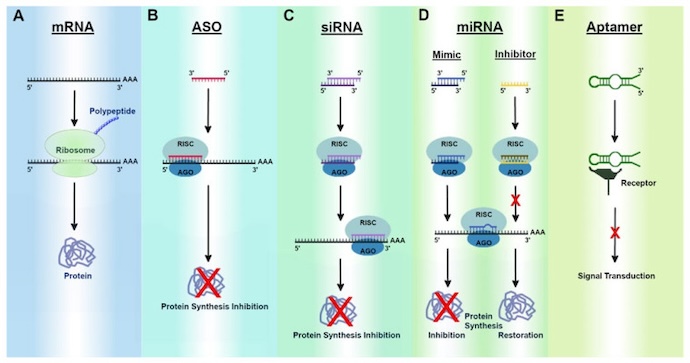
In this article, LifeSciencesIntelligence will focus on RNA-based therapeutics beyond mRNA vaccines.
Antisense Oligonucleotides (ASOs)
ASOs are short single-stranded nucleic acid polymers (oligonucleotides) that complement RNA sequences, altering the RNA and subsequent protein expression. There are two types of ASOs: occupancy-mediated degradation and occupancy-only models.
Occupancy-mediated degradation models bind ASOs to target RNAs using endogenous enzymes to downregulate transcripts through enzymatic RNA degradation.
Occupancy-only mechanisms bind ASOs to RNA without using specific enzymes to down or up-regulate target transcripts.
ASOs have shown promise as therapeutic agents for various diseases, including genetic disorders, neurodegenerative diseases, viral infections, and certain cancers.
There are multiple FDA-approved ASOs for several conditions:
- Fomivirsen: FDA-approved in 1998 to treat cytomegalovirus (CMV) retinitis
- Mipomersen: FDA-approved in 2013 for homozygous familial hypercholesterolemia
- Nusinersen: FDA approved in 2016 for spinal muscular atrophy
- Etiplirsen: FDA approved in 2016 for Duchenne muscular dystrophy
- Inotersen: FDA approved in 2018 for familial amyloid polyneuropathy
- Golidirsen: FDA approved in 2019 for Duchenne muscular dystrophy
- Milasen: FDA approved in 2018 for Mila Makovec’s CLN7 gene associated with Batten disease
- Casimersen: FDA approved in 2021 for Duchenne muscular dystrophy
RNA Interference (RNAi)
RNAi is a natural cellular process that can be harnessed for therapeutic purposes. Small RNA molecules, such as siRNAs or short hairpin RNAs (shRNAs), are introduced into cells to trigger the RNAi pathway. The small RNA molecules bind to target mRNA molecules, leading to their degradation or translational repression.
RNAi encompasses multiple types of RNA-based therapeutics, including siRNAs and microRNAs.
siRNAs are small double-stranded RNA sequences that silence gene expression and protein production. According to an article in the International Journal of Molecular Sciences, Dicer isolates siRNA from longer endogenous or viral precursor siRNAs, leaving a 3’ overhang that allows the siRNA to interact with the RNA-induced silencing complex (RISC).
“Once it is bound, the RISC protein argonaute 2 (AGO) carries out the cleavage of the sense strand. This allows for the antisense strand to bind its target mRNA. Once the target RNA is bound to the antisense strand, its phosphodiester backbone is cleaved by AGO2. This leads to sequence-specific knockdown of the target mRNA and therefore causes gene silencing,” noted researchers in the article.
The FDA has approved multiple siRNA treatments, including the following:
- Patisiran, which downregulates transthyretin to treat polyneuropathy caused by hATTR amyloidosis, was approved in 2018.
- In 2020, the FDA approved Givosiran to downregulate ALAS1 in patients with acute hepatic porphyria.
- In the same year that Givosiran was approved, Lumasiran, which downregulates glycolate oxidase in patients with hyperoxaluria type 1, was approved.
- Inclisiran downregulated proprotein convertase subtilisin/kexin type 9 to treat atherosclerotic cardiovascular disease and was approved in 2021.
Another RNA-based therapeutic category is microRNAs or miRNAs, which can inhibit or promote protein production, depending on its application. These small RNAas can bind to an mRNA target, preventing protein synthesis, or bind to other miRNAs that are repressing protein production to promote production.
Aptamer
Aptamers are short RNA or DNA molecules that can bind to specific target molecules, such as proteins or small molecules. They are selected through SELEX (Systematic Evolution of Ligands by Exponential Enrichment). Aptamers can interfere with their target molecules' activity by blocking their function directly or inducing a downstream effect.
In 2002, the FDA approved Pegaptanib, an aptamer that blocks VEGF-165 to treat neovascular age-related degeneration. Over a decade later, in 2020, the administration approved another aptamer, defibrotide, which activated adenosine A1/A2 receptors to treat veno-occlusive disease in the liver.
“While there are currently challenges to RNA therapeutic development, unprecedented interdisciplinary approaches and promising developments in modern science, along with improved early study design for clinical trials, will overcome these obstacles in the foreseeable future. This will provide substantial hope for the clinical utility of RNA therapeutics for different disease conditions and lead to a better quality of life for millions of patients,” concluded researchers in the International Journal of Molecular Sciences.





