StarLineArts/istock via getty im
Exploring Biofilms on Medical Devices and Intervention Strategies
Research sheds light on a hidden threat to the healthcare community and patients: biofilms.
In the ever-evolving healthcare community, advancements in medical devices have revolutionized patient care and treatment outcomes. However, the healthcare industry faces an ongoing threat: biofilms. These slimy, surface-attached communities of microorganisms have a significant impact on medical devices and infection control protocols, posing a threat to patient safety and treatment efficacy. Research has highlighted the importance of understanding and combating biofilms to improve patient outcomes.
What Are Biofilms?
Biofilms — an ancient biological phenomenon dating back to roughly 3.4 billion years ago — are complex communities of bacteria, fungi, and other microorganisms that form a protective matrix after adhering to any surface where the environment provides an abundance of proteins and other molecules.
One distinguishing characteristic of biofilms is the presence of extracellular polymeric substances (EPS) — primarily polysaccharides — that surround and encase the cells. These polysaccharides can be observed under scanning electron microscopy as thin strands connecting the cells and the surface or as sheets of amorphous material on the surface. Most of the biofilm's volume comprises this extracellular polymeric substance rather than cells, as confirmed by ruthenium red staining and transmission electron microscopy. The biofilm matrix can act as a filter, trapping minerals and host-produced serum components.
Biofilm Life Cycle
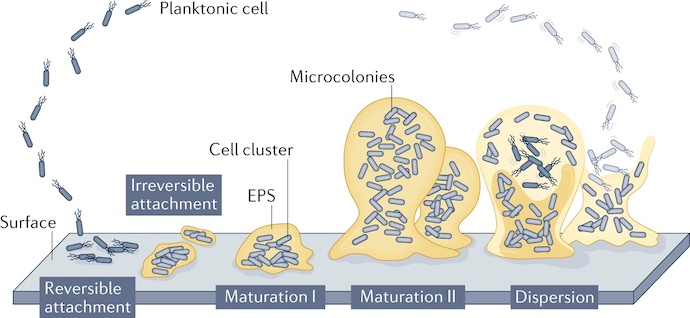
According to various sources, the biofilm life cycle involves at least four stages — attachment, microcolony formation, maturation, and dispersal/re-attachment.
Attachment
During the attachment stage, individual microorganisms, such as bacteria, algae, or fungi, attach themselves to a surface. This initial attachment process is often facilitated by the production of adhesion molecules and extracellular polymeric substances, which help form a protective matrix.
Microcolony Formation
Once attached, the microorganisms begin to multiply and form microcolonies. As they replicate, they start to secrete EPS, which provides structural support and acts as a glue-like substance to hold the biofilm together.
Maturation
The biofilm continues to grow and mature as more microorganisms join the colony. The EPS matrix becomes more complex and denser, creating a protective environment for the microorganisms. The biofilm reaches a steady state with balanced growth and detachment rates. Nutrient availability, environmental conditions, and microbial interactions contribute to maintaining the biofilm structure and composition.
Within the intricate and dynamic community of microorganisms within a mature biofilm, various interactions take place. One example is a mutual benefit, where one microbe can derive advantages from the waste products produced by another microbe.
Another interaction involves the transfer of genetic material within the EPS, which can occur between cells or different species. This exchange promotes functional adaptation of the microbial community within the biofilm.
By developing cooperative or competitive relationships, these microbes may contribute to reducing the efficiency of sanitization methods — eventually turning into a potential source of contamination.
Dispersal/Re-Attachment
According to researchers, numerous factors — such as nutrient levels, oxygen tension, pH, and temperature — have been shown to induce the dispersal of biofilms produced by various species. Biofilm dispersal allows the microorganisms to spread and colonize new surfaces and initiate the biofilm life cycle again, completing the process pictured below.
Dispersal mechanisms can include the release of enzymes or signaling molecules that break down the EPS matrix, the detachment of individual or small groups of microorganisms, or the shedding of biofilm fragments.
Impact of Biofilms on Medical Devices
Biofilms can be found on a variety of medical devices, such as catheters, implants, and prosthetics, leading to device-related infections. Biofilms can act as reservoirs for pathogenic microorganisms, allowing them to evade the body's immune system and resist the effects of antibiotics and sanitizers. Consequently, biofilm-related infections are notoriously difficult to treat and can lead to severe complications, prolonged hospital stays, and increased healthcare costs.
Biofilms found on medical devices can consist of Gram-positive or Gram-negative bacteria or yeasts. Commonly isolated bacteria from these devices include Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa.
These organisms can originate from the skin of patients or healthcare workers, tap water that the device's entry ports are exposed to, or other environmental sources. Depending on the device and how long it has been used in a patient, biofilms can be composed of a single or multiple species. In the case of urinary catheters, biofilms may start with a single species but eventually become multispecies biofilms with prolonged exposure, according to the CDC.
The prevalence of biofilm-related infections from medical devices has become a significant clinical concern. Because biofilms are known to be highly resilient and resistant to antimicrobial treatment and traditional antimicrobial strategies , understanding the mechanisms by which biofilms form and thrive has become a focal point for many researchers. This knowledge is crucial for developing strategies to prevent and treat biofilm-related infections.
Scientists have identified several key factors that contribute to biofilm formation, including surface characteristics of medical devices, the presence of organic materials, and the ability of microorganisms to communicate and cooperate within the biofilm structure. By targeting these factors, researchers hope to develop novel approaches to prevent biofilm formation or disrupt existing biofilms.
Preventative Measures
One promising area of research involves the development of antimicrobial coatings for medical devices. These coatings, often based on nanoparticles or novel materials, can prevent bacterial attachment and inhibit biofilm formation. By incorporating antimicrobial properties into the surface of medical devices, researchers aim to reduce the risk of device-related infections and improve patient outcomes.
In addition to preventive measures, researchers are exploring innovative treatment options for biofilm-related infections. Traditional antibiotic therapy often fails in eradicating biofilms due to their protective nature. However, alternative approaches such as antimicrobial peptides, bacteriophages, and biofilm-specific enzymes are being investigated for their potential to target and disrupt biofilms effectively.
The Role of Healthcare Providers
Healthcare providers and infection control teams play a vital role in combating biofilms. Implementing stringent infection control protocols, including proper device handling, regular surveillance, and early detection of biofilm-related infections, is crucial. Ongoing education and training on biofilm-related issues will ensure that healthcare professionals stay up-to-date with the latest research and best practices.
As the healthcare industry continues to advance, understanding the impact of biofilms on medical devices and infection control is paramount. With further research and collaboration between scientists, healthcare providers, and industry stakeholders, innovative solutions can be developed to combat biofilm-related infections effectively. By addressing this hidden threat, the healthcare community can enhance patient safety and improve outcomes, marking a significant milestone in the fight against healthcare-associated infections.