
Getty Images/EyeEm
Evaluating the safety profile of mifepristone
Evaluating the safety profile of mifepristone amid legal debates and access challenges underscores its importance in reproductive healthcare.
Mifepristone, commonly known as the abortion pill, is a medication used primarily for medically induced abortions as well as the management of Cushing's syndrome and uterine leiomyomas. Since its approval in 2000, mifepristone has been the subject of extensive research and scrutiny, although it has a favorable safety profile.
The safety evaluation of mifepristone has gained significance due to its connection to ongoing legal and political debates surrounding abortion. The United States Supreme Court's consideration of the FDA's evaluation has implications for access to medication abortions and the future of reproductive rights. Because public opinions on abortion vary, the controversy surrounding the topic influences discussions on the safety and accessibility of medications like mifepristone.
Understanding Mifepristone
In medically induced abortions, mifepristone — a synthetic steroid, also known as RU-486 — is a competitive progesterone antagonist that binds to its intracellular receptor at low doses. At high doses, mifepristone also blocks cortisol at the glucocorticoid receptor, leading to increased circulating cortisol levels, which helps control hyperglycemia in patients with Cushing's syndrome.
Mifepristone is an antiprogestin that blocks progesterone, a natural steroid hormone that prepares the uterus for embryo implantation. By inhibiting progesterone's action, mifepristone causes the uterus lining to thin and prevents the embryo from attaching to the uterine wall. The medication is typically used with misoprostol, a prostaglandin analogue that induces contractions, leading to the embryo's expulsion.
Mifepristone is also used as an emergency contraceptive following unprotected sexual intercourse (known as the morning-after pill); for addressing specific conditions like a certain type of brain tumor, endometriosis (the proliferation of uterine tissue beyond the uterus), or fibroids (noncancerous growths in the uterus); or to initiate labor (aiding in commencing the birthing process in a pregnant woman).
Mifepristone is available as an oral tablet in 200 mg and 300 mg preparations. The dosing varies depending on the indication. For pregnancy termination, a single oral dose of 200 mg is typically administered. However, in managing hyperglycemia in Cushing's syndrome, an initial dose of 300 mg is given orally once daily, possibly increasing the dose to 1200 mg daily. Different dosing regimens apply to other indications.
Safety and Efficacy
Extensive clinical trials and real-world data have demonstrated that mifepristone has a favorable safety profile when used according to recommended protocols. The World Health Organization (WHO) and various medical organizations recognize it as a safe and effective method for terminating early pregnancies.
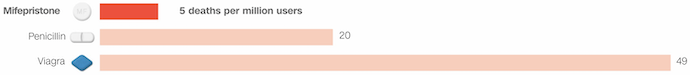
The safety profile of mifepristone stands out remarkably compared to other commonly prescribed medications. According to data analyzed by CNN, mifepristone's safety record is impressive, with only five deaths associated with its use for every 1 million people in the US who have used the drug since its approval in 2000. This translates to an incredibly low death rate of 0.0005%.
To put this into perspective, mifepristone's safety surpasses that of some widely used prescription drugs. For example, the risk of death associated with mifepristone is four times lower than penicillin, a common antibiotic used to treat bacterial infections like pneumonia.
Additionally, the risk of death by taking Viagra, a medication used to treat erectile dysfunction, is nearly ten times higher than the risk associated with mifepristone (Figure 1).
Figure 1. Comparative Safety Analysis
These statistics underscore the established safety profile of mifepristone, which over five million individuals have utilized for more than two decades. Such a safety record is crucial, especially given the sensitive nature of its use in terminating pregnancies. The data suggests that mifepristone is not only effective but also remarkably safe, offering reassurance to individuals considering its use for medication abortion or other approved medical purposes.
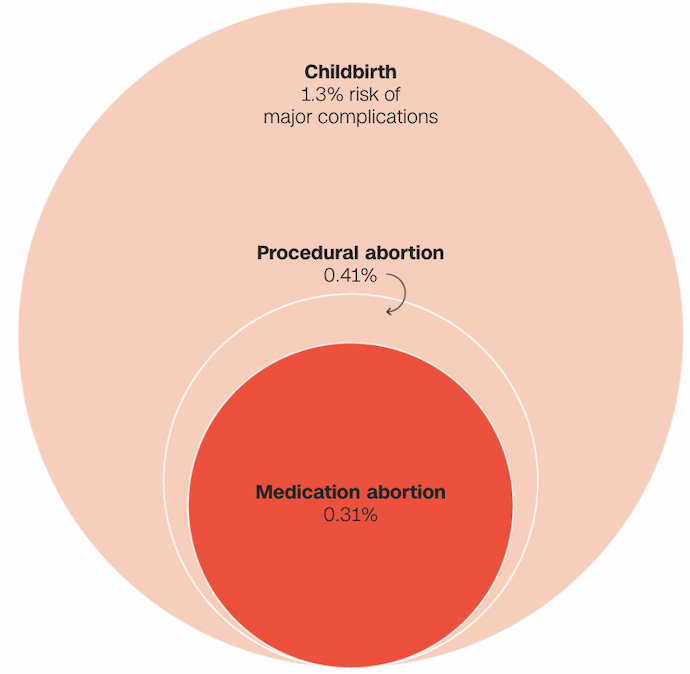
Data suggests that the likelihood of experiencing severe complications is greater with childbirth and procedural abortion compared to medication abortion (Figure 2).
Figure 2. Comparative Safety of Medication Abortion, Procedural Abortion, and Childbirth
Since its introduction, the FDA has continuously monitored the safety of mifepristone, expanding its approved usage based on available data. Initially approved for pregnancies within the first seven weeks, the FDA extended the window to 10 weeks in 2016. Furthermore, the COVID-19 pandemic accelerated the acceptance of telemedicine, leading the FDA to waive the in-person requirement for mifepristone prescriptions in 2021. This change allowed patients to obtain the medication through remote consultations with healthcare providers, eliminating significant barriers to abortion access.
Various medical studies and extensive data support the safety and efficacy of mifepristone within the two-drug regimen. In a friend-of-the-court brief filed on behalf of the FDA and Danco Laboratories, major medical societies, including the American College of Obstetricians & Gynecologists and the American Medical Association, stated that major adverse events occur in less than 0.32% of patients, with an almost non-existent risk of death.
Mifepristone's safety has been extensively studied across various populations, including adolescents, women with chronic medical conditions, and those with previous cesarean sections or ectopic pregnancies. Research indicates that mifepristone can be safely administered to these populations, with similar effectiveness and safety outcomes.
Long-Term Safety
Studies evaluating the long-term safety of mifepristone have not found any significant adverse effects on future fertility, ectopic pregnancy rates, or the occurrence of birth defects in subsequent pregnancies.
Regarding reported deaths associated with mifepristone usage, the FDA's data indicates uncertainty about the pill's direct responsibility. Out of 32 deaths reported between September 2000 and the end of 2022, mifepristone's role in these fatalities remains unclear. Some deaths were attributed to factors such as ruptured ectopic pregnancies, drug intoxication, overdoses, or homicides. Medical groups supporting the FDA and Danco Laboratories estimate that, in the worst-case scenario, mifepristone might be responsible for no more than 13 out of the 32 deaths, suggesting a lower risk compared to other commonly used medications.
Potential Side Effects
Like any medication, mifepristone can have side effects. The most common ones include abdominal pain, cramping, nausea, vomiting, diarrhea, and bleeding. However, these side effects are generally temporary and resolve without medical intervention. Serious complications, such as excessive bleeding or infection, occur rarely and can be managed effectively with appropriate medical care.
While the majority of patients experience mild side effects such as headaches, weakness, and nausea, it is crucial to be aware of potential serious complications. These include heavy bleeding, severe abdominal pain, fever lasting more than four hours, and persistent sickness after taking misoprostol. These symptoms may indicate a life-threatening infection, an ectopic pregnancy, or another serious issue requiring immediate medical attention.
Legal and Access Considerations
Access to mifepristone varies across countries due to legal and regulatory restrictions. Expanding access to safe abortion care, including mifepristone, is crucial for ensuring reproductive rights and reducing maternal mortality and morbidity associated with unsafe procedures.
Mifepristone has demonstrated a favorable safety profile when used as advised. While the medication may have temporary side effects, serious complications are rare and can be effectively managed with appropriate medical care. Ensuring access to mifepristone and comprehensive reproductive healthcare is vital for promoting reproductive health, autonomy, and well-being.





