
Getty Images
Evaluating the Series of Infant Formula Recalls in the US
PharmaNewsIntelligence explores the various stages of infant formula recalls in the US and how the FDA has adapted to minimize contamination.
Infant formula plays a critical role in pediatric healthcare, providing an alternate route for nutrition in babies who do not breastfeed or may need more nutrients than what is provided in breast milk. Although many people, including health experts, maintain that breastfeeding is the best nutritional choice for most infants, breastfeeding all the time may not be possible in many cases.
In an article for Nemours KidsHealth, Elana Pearl Ben–Joseph, MD, primary care pediatrician at Nemours Children’s Health, revealed that multiple factors may lead a parent or guardian to opt for formula feeding, including comfort, latch-on pain, time and frequency of feedings, diet, and maternal medications or health conditions.
Formula feeding can be more convenient for some families and caregivers, providing additional flexibility.
Theoretically, infant formula can be a sterile nutritional alternative to breast milk, and it is sometimes fortified with additional nutrients that some infants cannot get with breast milk. However, as demonstrated by a recent flurry of formula recalls and shortages, the sterility and availability of formula may be compromised by manufacturing flaws.
Original FDA Oversight of Infant Formula
According to the FDA infant formula resources page, the FDA monitors infant formula's safety, nutritional adequacy, packaging, and labeling; however, the administration does not have the authority to approve or deny formulas on the market.
Conversely, the FDA is authorized to require a mandatory recall if an adultered or misbranded formula poses a health risk.
Beyond assessing the nutrients of infant formula, requiring a minimum amount of 30 different nutrients and issuing maximum amounts of 10 additional nutrients, the FDA also has a set of guidelines for minimizing the risk of contamination.
The FDA notes, “Controls are required by law to prevent contamination and other problems from happening. For example, manufacturers must establish a system of controls designed to ensure that infant formula does not become adulterated due to the presence of microorganisms in the formula or the processing environment.”
However, the baseline strategies for preventing contamination and bolstering the supply chain have failed to do their job effectively. Since the series of recalls has compromised the supply chain and infant health, the FDA has issued updated guidelines and policies to protect consumers further.
A Brief Timeline of the Recalls
Before diving into updated policies, it is critical to understand the series of recalls that have contributed to these changes.
Although the infant formula supply chain, like many other supply chains, was compromised by the COVID-19 pandemic, recalls starting in February 2022 perpetuated the shortage further.
Below is a timeline of infant formula recalls caused by bacterial contamination:
- 02/17/22: Abbott Recalls Similac, Alimentum, and EleCare formulas manufactured in Sturgis, Michigan, due to Cronobacter sakazakii or Salmonella Newport
- 02/28/2022: Abbott adds a batch of Similac PM 60/40 to the recall list due to Cronobacter sakazakii
- 12/11/2022: ByHeart Whole Nutrition Infant Formula Recalled due to Cronobacter sakazakii
- 02/20/2023: Reckitt Recalls Enfamil Prosobee Simply Plant-Based Infant Formula in 12.9 oz containers due to Cronobacter sakazakii
- 03/17/2023: Gerber Good Start SootheProTM Powdered Infant Formula recalled by Perrigo due to potential Cronobacter sakazakii
- 05/13/2023: Associated Wholesale Grocers updates Gerber Good Start SootheProTM Powdered Infant Formula recall
- 12/31/2023: Enfamil Recalled by Rekitt and Mead
Although there have been other infant formula recalls due to undeclared milk and recalls on liquid formulations. PharmaNewsIntelligence will focus on infant formula powder recalled for contamination issues, as they make up most recalls.
Abbott
The first of many infant formula recalls came from Abbot Laboratories, one of the largest infant formula manufacturers in the United States. On February 17, 2022, Abbott voluntarily recalled three infant formula brands manufactured in Sturgis, Michigan.
According to the company’s statement, published on the FDA website, “The products under recall have a multidigit number on the bottom of the container starting with the first two digits 22 through 37, contains K8, SH, or Z2 and with an expiration date of April 1, 2022, or after.”
This set of recalls followed reports from four consumers linking these infant formulas to bacterial infections. Three consumers linked the infant formula to Cronobacter sakazakii, while one mentioned Salmonella Newport.
The initial release from Abbott maintained that the bacteria were not detected in any of the samples tested by the manufacturer; however, Joe Manning, Abbott's executive vice president of nutritional products, stated, “We're taking this action so parents know they can trust us to meet our high standards, as well as theirs. We deeply regret the concern and inconvenience this situation will cause parents, caregivers, and healthcare professionals."
Shortly after the initial recall, on February 28, 2022, Abbott expanded the recall to add two lots of Similac PM 60/40 formula, lot numbers 27032K80 (can) and 27032K800 (case), also manufactured in the Michigan facility.
ByHeart
Throughout the remainder of 2022, infant formula recalls slowed until December 11, 2022, when ByHeart recalled five batches of ByHeart Whole Nutrition Infant Formula due to potential cross-contamination with Cronobacter sakazakii.
The recalled batches included the following: 22273 C1, 22276 C1, 22277 C1, 22278 C1, and 22280 C1. However, the company, like Abbott, emphasized that none of the samples had tested positive at the time of the recall.
Beyond that, ByHeart noted that it had not received any consumer complaints and did not believe the recall was connected to its manufacturing facilities. Instead, they attributed the recall to a third-party facility they contracted out for the final step of the manufacturing procedure, canning.
Reckitt
In February 2023, Reckitt followed suit, issuing its own voluntary recall of two batches of Enfamil ProSobee 12.9 oz Simply Plant-Based Infant Formula due to potential Cronobacter sakazakii cross-contamination, including batches ZL2HZF and ZL2HZZ both with a UPC Code of 300871214415. These two lots accounted for nearly 150,000 cans of formula.
Again, the company maintained that their products tested negative and no adverse reactions have been reported.
“We are committed to the highest level of quality and safety, and it is for this reason that we have taken this extraordinary measure. The batches in question tested negative for Cronobacter and other bacteria, and this is an isolated situation. After a thorough investigation, we have identified the root cause, which was linked to material from a third party. We have taken all appropriate corrective actions, including no longer sourcing this material from the supplier,” the company wrote in the recall notice.
Most recently, Reckitt and its subsidiary, Mead Johnson Nutrition, issued a voluntary recall of Enfamil Nutramigen Powder infant formula in 12.6 and 19.8 oz cans on December 30, 2023. The recall applied to the following lots: ZL3FRW, ZL3FPE, ZL3FXJ, ZL3FQD, ZL3FMH, ZL3FHG with a UPC of 300871239418 or 300871239456.
Perrigo
Between the two recalls from Reckitt, on March 17, 2023, Perrigo Company PLC voluntarily recalled Gerber Good Start SootheProTM Powdered Infant Formula in the 12.4, 30.6, and 19.4 oz sizes. The recalled products include the following lot numbers:
- Gerber Good Start SootheProTM 12.4 oz: 300357651Z, 300457651Z, 300557651Z, 300557652Z, 300757651Z, 300857651Z, 301057651Z, 301057652Z, and 301157651Z
- Gerber Good Start SootheProTM 30.6 oz:
301357652Z, 301457652Z, and 301557651Z - Gerber Good Start SootheProTM 19.4 oz: 301557652Z
Although none of the tested samples contained Cronobacter, the company recalled the product due to potential contamination.
A few months later, Associated Wholesale Grocer issued their own recall notice on the Gerber Good Start SootheProTM sold at its retailers.
Contamination Risks
Beyond evaluating the series of recalls to justify the policy changes, it is critical to assess the impact of contamination on human health.
According to the CDC, Cronobacter sakazakii, commonly referred to as Cronobacter, is a Gram-negative, coliform bacteria known for its ability to survive in arid environments, including dry foods, such as infant formula, powdered milk, starches, and more.
Although Cronobacter can impact people of all ages, the highest risk is among infants. CDC data suggests that Cronobacter can cause sepsis and meningitis in infants, with the highest incidence in infants two months or younger.
In fact, the CDC notes that Cronobacter-related meningitis is nearly exclusive to that age range. Once an infant has Cronobacter-related meningitis, the risk of death in the US is 20%. Globally, that risk doubles to roughly 40%.
FDA Policy Changes
Considering the potentially fatal risks associated with infant formula contamination, the FDA has taken steps to minimize the risks of contaminated products and guide manufacturers' adoption of more sterile and safe manufacturing procedures.
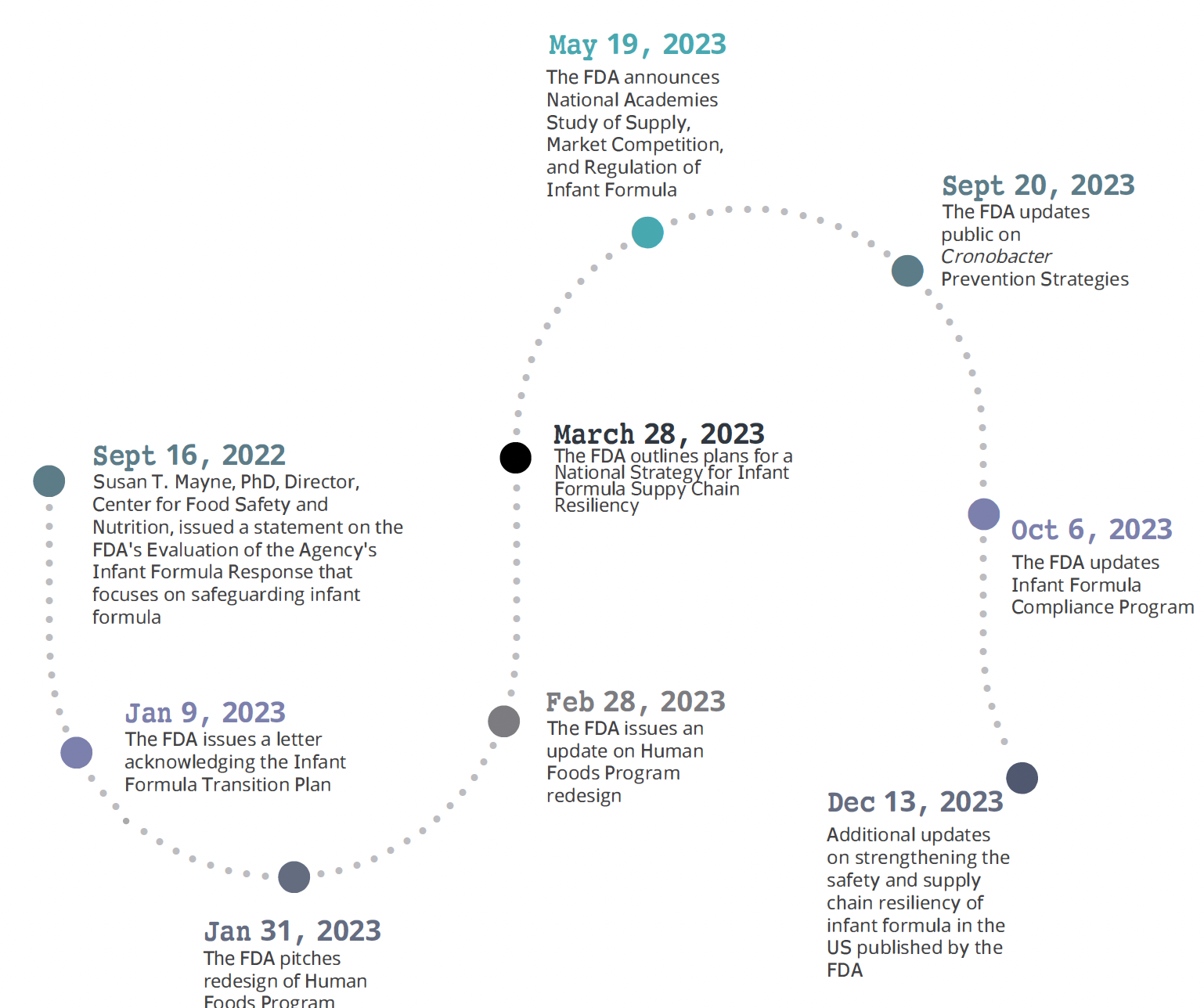
Below is a timeline outlining the updates made by the FDA:
- 11/16/2022: Susan T. Mayne, Ph.D., Director, Center for Food Safety and Nutrition, issued a statement on the FDA’s Evaluation of the Agency’s Infant Formula Response that focuses on safeguarding infant formula
- 01/09/2023: FDA issues a letter acknowledging the Infant Formula Transition Plan
- 01/31/2023: FDA pitches redesign of Human Foods Program
- 02/28/2023: FDA issues update on Human Foods Program redesign
- 03/28/2023 FDA outlines plans for a National Strategy for Infant Formula Supply Chain Resiliency
- 05/19/2023: FDA Announces National Academies Study of Supply, Market Competition, and Regulation of Infant Formula
- 09/20/2023: FDA updates public on Cronobacter Prevention Strategies
- 10/6/2023: FDA updates Infant Formula Compliance Program
- 12/13/2023: Additional updates on strengthening the safety and supply chain resiliency of infant formula in the US published by FDA
As the FDA continues to adapt its guidelines for infant formula monitoring, parents, consumers, and providers should closely monitor the FDA recall notice page to evaluate the safety of their products.







