
metamorworks - stock.adobe.com
Effectively Managing Clinical Trials, Recruitment, and Adaptive Trials
Learning how to effectively manage clinical trials, from recruitment to trial design and adaptability, is critical for the economic well-being of clinical research organizations.
Clinical trial significance and success throughout the COVID-19 pandemic have inspired the launch of clinical trial branches for existing companies, such as Walgreens, and multiple startups. However, it is critical to note that while some clinical trials have public and widely recognized success, most are unsuccessful. Successful clinical research organizations have learned how to effectively manage clinical studies from all fronts, developing clear recruitment strategies and adapting trials as needed.
Clinical Trial Success Rates
As science advances, the clinical trial industry continues to grow and develop. The global market was valued at roughly $47 billion in 2021. A report by Grand View Research estimates an annual growth rate of 5.8% from 2022 to 2030.
Despite significant economic growth, the FDA notes that only 70% of drug trials move from Phase 1 to Phase 2. Roughly one-third of trials move from phase 2 to phase 3, while approximately 25–30% move from phase 3 to phase 4. This data suggests that roughly 90% of clinical trials fail.
From a scientific standpoint, failure is a necessary step toward success. A failed clinical trial can help clinicians and healthcare professionals gather the information that may provide background for later clinical trials. However, from a business perspective, clinical trials require funders and organizations to take considerable financial risk. Investors must understand the clinical business model and best practices to ensure the most significant financial success when deciding whether to back or start a clinical trial.
Factors to Consider in Clinical Trial Management
Based on a presentation from the University of Alabama Birmingham, three main areas of management are required in the business of clinical trials: program management, clinical trial management, and financial management. Understanding these management areas can better equip companies for a successful clinical trial.
Program Management
Managing the program involves hiring the teams in charge of the clinical trial and training them. This process is relatively easy for many established clinical research organizations (CROs) and pharmaceutical companies. Pharmaceutical companies already have their own pharmacists, healthcare professionals, engineers, and technicians on staff with some pre-established knowledge of the company’s practice.
Other factors to consider when managing the program include equipment, maintenance, supplies, and the development of a performance portfolio to assess the program’s efficacy.
Clinical Trial Management
In addition to managing the program as a whole, another critical aspect is actually managing the clinical trial. This often requires an in-depth feasibility assessment of all parts of the study. It provides leaders with information on the project's scope, how likely the company is to complete the project successfully, and how likely it is to meet the end goal. Below is a graphic from Applied Clinical Trials that details the varying components of the feasibility process.
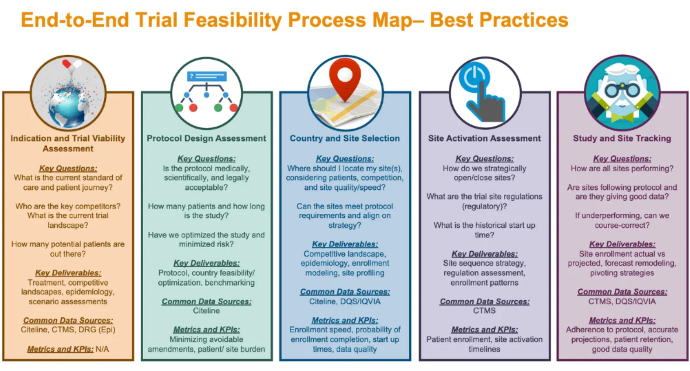
In addition to conducting feasibility assessments, the CRO should also consider conducting an analysis of interest, answering the following question: are there enough potential participants interested in this trial? Assuming the answer is yes, the CRO can begin the patient recruitment process in collaboration with the clinical trial site.
The final portion of managing the actual clinical trial is understanding and planning for lack of success. As previously mentioned, most clinical trials are unsuccessful, meaning that the time, money, and work put into many clinical trials go wasted. Part of effectively managing a clinical trial from a business perspective is knowing when unsuccessful clinical trials should be discontinued and when they can be adjusted or repurposed. To mitigate wasted money, many companies are turning toward adjustable clinical trials to redirect efforts when early studies prove to be futile.
Money Management
The final business aspect of managing clinical trials is learning how to manage the finances associated with them. There are multiple costs related to clinical trials, including indirect fees paid to the clinical trial site or institutions conducting the study, patient care costs, personnel time, document storage, FDA audits, and more.
Best Recruiting Practices
As previously mentioned, part of a clinical trial’s success relies on recruiting the appropriate patient population. Recruitment strategies can vary dramatically depending on the intended therapeutic area, the age of patients, and the type of study — interventional or observational.
More often, recruiters are looking toward digital media recruitment strategies. While recruitment depends on the clinical trial site, an Antidote article lists some best practices for patient recruitment.
Chief of this recommendation is a patient-centric approach to recruitment. As with any part of clinical trials, a focus on the patient or types of patients in this trial is critical. When advertising for clinical trial recruitment, it is essential to answer any questions a patient may have clearly and easily. Leaving questions unanswered can hold patients back from pursuing the trial. In the same vein, another best practice is to plan for advertisements that reach the target audience, excluding or including recruitment strategies based on participant criteria.
Another vital practice during recruitment is ensuring that all advertisements follow the platform's protocols, policies, and regulations. Advertisements typically require a financial contribution, which typically pays off by recruiting several patients. However, they can be taken down if the ads do not follow policies.
Analyzing the recruitment campaign is also crucial for ensuring that clinical trials stay on track business-wise. Funneling money into specific recruitment methods without analyzing their success is foolish. Companies who have previously done clinical trials may choose to assess the success of previous recruitment tools. In contrast, new companies may consider analyzing the campaign results halfway through recruitment.
Finally, researching and understanding new digital recruitment platforms and tools is critical for clinical trial leaders focused on the recruitment process.
Understanding Adaptive Clinical Trials
With a failure rate of nearly 90%, clinical trials lead to enormous research and development spending. Standard practice in the clinical trial landscape is to have a study protocol developed before its start and remain on the pre-determined pathway throughout the study, barring any safety concerns. Should the study produce unfavorable results, it would be discontinued. However, this rigid structure prevents clinical trials from being adaptive and able to accommodate unanticipated changes.
One proposed solution for reduced clinical trial spending is transitioning to an adaptive clinical trial model. According to Amgen, one of the best ways to improve clinical trial profitability is transitioning from a fixed to a flexible clinical trial design. "With adaptive designs, you can monitor the incoming data and modify the protocol based on what you're learning as the study unfolds," noted Rob Lenz, senior vice president of Global Development of Amgen, in the article.
This doesn’t mean allowing any changes to be made at any point during a clinical trial. Instead, it identifies predetermined conditions that could indicate an adaption. Some examples provided by Amgen include dosage changes depending on efficacy data, trial size or duration changes to understand the impact better, or adding more patient types.
Another critical goal for clinical trial development and improvement is to improve the success rate of trials while reducing costs. In an article published by Amgen, the organization notes, “In conventional clinical development programs, tradeoffs are required if you want to prioritize speed, cost, or likelihood of success. To optimize for success, you often accrue more data at each stage of the program, but that makes the program go slower. Optimizing for cost by staging your investment also slows you down, while optimizing for speed tends to drive up the cost. With adaptive designs, it's possible to simultaneously increase the odds of success, reduce spending, and get to an answer more quickly.”
Instead of sticking to an incorrect assumption made before the start of a clinical trial or discontinuing a trial altogether due to false beliefs, adaptive clinical trials allow clinicians and researchers to correct some inaccurate assumptions and continue with the study.






