
Prostock-studio - stock.adobe.co
Considerations in Pediatric Clinical Trials, Ensuring Patient Safety
Pediatric clinical trials have the potential to address many unmet needs and gaps in healthcare; however, contemplating the many considerations in pediatric clinical trials is essential for mitigating concerns and ensuring patient safety.
The clinical trial landscape — globally and in the United States — is one of many complexities, difficulties, implications, and guidelines. Clinical trials can provide critical information on treating chronic and acute illnesses. When approving medications, devices, and more, the FDA often looks to data from clinical trials to make informed, data-driven regulatory decisions. Often, many people fail to recognize the role of pediatric participants in clinical trials. Considering the comparatively increased complexity and opacity of pediatric healthcare, pediatric participation in clinical trials requires extensive consideration by industry leaders, providers, and parents. Understanding best practices in pediatric clinical trials are essential for ensuring favorable healthcare outcomes, mitigating concerns, and ensuring patient safety.
Pediatric Clinical Trial Market
Data from BMC Pediatrics notes that between 2008 and 2019 approximately 53,060 clinical studies related to children were registered on ClinicalTrials.gov. About 68.1% of these studies were interventional, 31.5% were observational, and 0.4% were expanded access studies.
According to a report from Future Marketing Insights, the pediatric clinical trial market was valued at $15.8 billion in 2022, relatively close to the $15 billion value of 2021. However, projections anticipate that the market value will increase by over $10 billion, reaching $26.47 billion, by 2032.
The report identifies various factors expected to drive the market growth, including unmet needs in pediatric populations across the United States. Other factors contributing to this growth include increased chronic disease incidence rates and shifting clinical trial landscapes, which have a broader range of trial sites and participants.
Need for Pediatric Clinical Trials
Healthcare professionals and the pharmaceutical industry are acutely aware of the differences in disease presentation and outcomes between children and adult populations. This remarkable difference in most cases means clinical trial results from adult participants cannot be applied to pediatric populations.
The WHO notes, “Children are a unique population with distinct developmental and physiological differences from adults. Clinical trials in children are essential to develop age-specific, empirically verified therapies and interventions to determine and improve the best medical treatment available.”
Components and Considerations of Pediatric Clinical Trials
A paper published in Clinical and Translational Science notes that approximately 20% of pediatric clinical trials fail due to inappropriate study design, suboptimal planning, or inadequate enrollment. Due to the complexity of pediatric clinical trials, many components and considerations must be considered when designing or conducting a pediatric clinical trial.
Legal Considerations
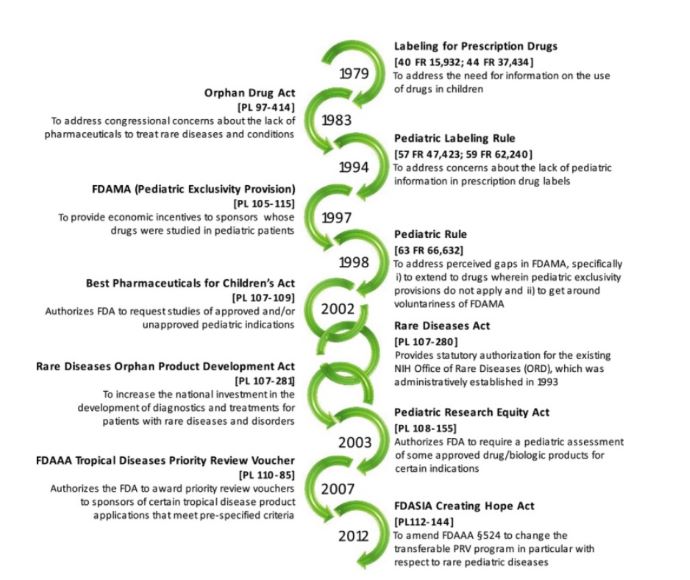
Over the years, the FDA and other regulatory healthcare organizations have advocated for and implemented many legislations to protect pediatric populations in pharmaceutical research and encourage extensive efforts to advance pediatric healthcare. Below is a timeline of legislative measures up to 2012.
Prospective Patient Population
In 2022, the FDA issued a draft guidance regarding pediatric clinical trial considerations. As part of the guidance, the FDA emphasized some clinical pharmacology considerations. Chief of these considerations is identifying the appropriate pediatric population to study. Clinical trial leaders may opt for one or more pediatric patient populations based on the disease, drug profile, scientific and ethical justifications, and developmental changes in pediatric populations.
Pediatric patient populations can be divided into four categories, including the following:
- neonates, birth to 1 month
- infants, 1–24 months
- children, 2–12 years
- adolescents, 12–17 years
Pharmacological Considerations
Pharmacokinetics is essential to consider when conducting clinical trials on pediatric populations. Many aspects of pharmacokinetics — including absorption, distribution, excretion, protein binding, and clearance — vary in pediatric populations significantly more than in adult populations.
Other pharmacological considerations include pharmacodynamics and pharmacogenomics, which should be considered across all clinical trials but are especially important when looking at pediatric populations.
Medication Formulation and Dosage
An article published in Clinical and Translational Science provides a tutorial on best implementing pediatric clinical trials. To start, researchers in this publication echo concerns of many pediatric experts, noting that protocols set up to be indistinguishable from adult protocols for the same treatment pose many problems.
A successful pediatric clinical trial begins far before recruitment as a part of the study design. The first consideration in designing a pediatric clinical trial starts with medication administration. Researchers in Clinical and Translational Science commented, “In a population where items intended for oral ingestion, including foods and medicines, are often rejected on the basis of smell, taste, and texture, the importance of drug formulation cannot be overemphasized.”
Clinical trial designers and scientists must consider age-appropriate formulations that ensure consistent and reliable drug delivery. Trial designers and sponsors may consider allowing multiple formulations of the tested medication to widen the potential pediatric patient population.
Additionally, dosing approaches require attention from the clinicians in charge of the trial. Dosing should account for wide variations in weight and body mass. Considering these variations and the differences in the rate of development, clinical trials for pediatric populations should reconsider fixed-dose approaches.
“Given the wide range in body size encountered in the pediatric population, the fixed‐dose approach may result in significant variability in weight‐adjusted or size‐adjusted drug exposures, which will need to be addressed during data analysis. Ultimately, the objective of the study, as well as the availability of an age‐appropriate drug formulation, should guide the dose selection strategy for a pediatric investigation,” stated researchers in Clinical and Translational Science.
Ethical Considerations
There are some ethical considerations regarding pediatric clinical trials as well. The first ethical consideration is informed consent and assent. In adult trials, informed consent is relatively straightforward, requiring the participant to provide it on their own behalf. However, in pediatric trials, a legal guardian grants informed consent by proxy. In addition to the legally required parental or guardian consent, there is also an ethical obligation to obtain participant assent before enrolling them in a clinical trial. Typically, patients without cognitive impairment can begin to provide assent (or dissent) at seven.
In addition, the US allows clinical trial leaders to provide compensation for participation in clinical trials. However, in some cases, compensation may border on coercion. Clinical and Translational Science recommends that “to avoid undue influence while still demonstrating appreciation for study engagement, the payment type and value ought to reflect the effort and commitment required on the part of the study participant first and the parent/guardian second.”
Furthermore, another consideration is monitoring and safety. Clinical trials for all populations, but especially for pediatric populations, should have clear and effective protocols to ensure patient safety and adequate monitoring.
“Children need access to safe and effective medical products, and healthcare professionals need data to make evidence-based decisions when treating children. However, children are a vulnerable population who can’t provide consent for themselves and are afforded additional safeguards when participating in a clinical investigation,” said Dionna Green, MD, director of the FDA’s Office of Pediatric Therapeutics, in an FDA press statement. “The best way to provide children with safe and effective treatment options is by including them in clinical research and providing these additional safeguards to protect them during clinical trials.”






