CRISPR Genome-Editing Technology: The Future of Gene Editing
CRISPR genome-editing technology has revolutionized genetic engineering by introducing the ability to alter an organism’s DNA to precision medicine.
CRISPR-Cas9 genome-editing technology makes it easy to disrupt a targeted gene or insert a new sequence at a precise location using a DNA template. Modifying specific DNA sequences in a gene without affecting other genes in a genome establishes an easy method for identifying the association between a particular gene and the organism.
Because prior genome-editing methods are labor-intensive, expensive, and less effective, CRISPR-Cas9 is gaining increasing popularity in the scientific community. This system can be easily tailored to any targeted DNA sequence with unparalleled specificity. Because the CRISPR-Cas9 system is easily derived from bacteria, producing the required proteins is inexpensive and easy.
What Is CRISPR-Cas9?
Clustered regularly interspaced short palindromic repeats (CRISPR) are bacterial DNA sequences copied from bacterial viruses (bacteriophages). CRISPR-associated (Cas) enzymes utilize bacteriophage DNA to screen all DNA sequences inside the bacterial cell continuously. An innate immune response is activated if the Cas enzymes recognize a matching CRISPR sequence, which destroys the invading viral DNA and prevents bacteriophage infection.
Geneticists and medical researchers use CRISPR-Cas9 to edit portions of the genome by adding, removing, or altering parts of the DNA sequence within bacteria, plants, and animal models.
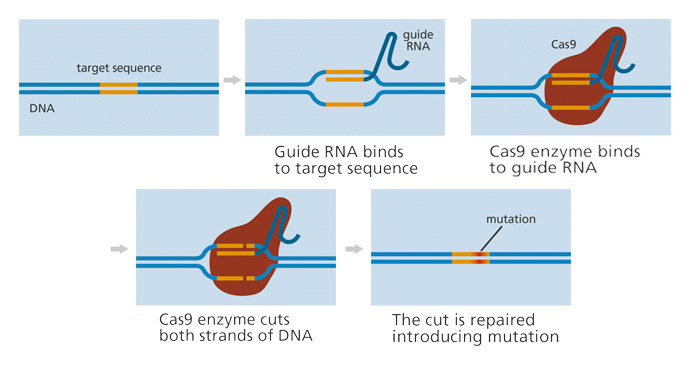
The mechanism responsible for CRISPR/Cas-9 genome editing consists of three steps summarized by recognition, cleavage, and repair, which relies on the combination of two vital molecules to modify targeted cells within a genome: guide RNA (gRNA) and CRISPR-associated protein 9 (Cas9).
Guide RNA (gRNA)
gRNA is a small pre-designed RNA sequence (around 20 bases long) embedded within a longer RNA scaffold intended to bind to the target sequence. gRNA is designed to guide Cas9 to the exact DNA sequence location to cut both DNA strands of interest.
In theory, because gRNA comprises complementary RNA bases, gRNA should only attach to the targeted cellular DNA sequence. Therefore, no other regions of the genome should be affected. The gRNA finds and binds to a specific, targeted DNA sequence.
CRISPR-Associated Protein 9 (Cas9)
Cas9 is a protein within the innate CRISPR system, which identifies, precisely cuts, and uniquely degrades the DNA of viral pathogens. In CRISPR-Cas9, the genetic engineering function of Cas9 has been manipulated to precisely insert or remove specific DNA fragments from a strand of genetic material, acting as a pair of molecular scissors.
How Was CRISPR-Cas9 Developed?
This groundbreaking technology emerged via basic research intended to uncover how bacteria use their adaptive immune systems to combat viral infections. In 2012, a small group of researchers theorized that CRISPR found in the genomes of prokaryotic organisms, including bacteria and archaea, could be reprogrammed to alter eukaryotic DNA.
The 2020 Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier, University of Umea, and Jennifer Doudna, University of California, Berkeley, for developing the genetic scissor mechanism popularly known as CRISPR-Cas9.
Since then, both women have battled against Feng Zhang of the Massachusetts Institute of Technology (MIT), who submitted a paper in October 2012, which reported the first successful programmable genome-editing tool and the first successful use in mammalian cells of CRISPR-Cas9.
After a lengthy legal battle over the commercial application of CRISPR, the US Patent and Trademark Office (PTAB) ruled in 2022 that the revolutionary CRISPR gene-editing technology belongs to the Broad Institute of Harvard and MIT.
Diversity of CRISPR Applications
CRISPR-Cas technology is rapidly becoming mainstream in numerous research applications such as those used in cancer research, genetic disorders, viral infections, and pathogen detection.
Agriculturally, CRISPR-Cas technology can develop desirable crop traits (i.e., herbicide tolerance and disease and pest resistance) and increase crop yield and quality.
Regulation and Limitations of CRISPR
Because CRISPR technology could be used to enhance desirable heritable traits, many researchers anticipate the implantation of ethical and international guidelines. While gene-editing technology is regulated differently in each country, researchers have called for a moratorium on heritable genome editing until full societal and ethical implications are determined.
Because many CRISPR-based treatments for conditions such as cancer, blood diseases, HIV/AIDS, Huntington’s disease, high cholesterol, muscular dystrophy, blindness, cystic fibrosis, and COVID-19 are in early-stage clinical trials, CRISPR treatments are probably years away from gaining FDA approval.
Although CRISPR-Cas9 is a potent tool for genetic disorders, clinical scientists struggle to deliver CRISPR-Cas9 material to mature cells on a large scale. Also, because some cells may lack genome-editing activity, CRISPR-Cas9 is not always 100% effective or 100% accurate since off-target edits can occur on rare occasions.
In the vast world of biology, gene-editing technology like CRISPR-Cas9 continues to change the face of biomedical research with unprecedented precision and ease with the potential of irradicating or destroying complex diseases for generations to come.





