
Rasi Bhadramani/istock via Getty
Assessing the Expected Impacts of OTC Hormonal Birth Control Pills
Newly approved OTC hormonal birth control pills are expected to improve contraceptive access, reduce healthcare spending, and save time.
Across the United States, access to reproductive healthcare services has been compromised due to state-level policy changes after the US Supreme Court overturned Roe v. Wade in the Dobbs case. Despite access disparities, the approval of an OTC birth control method has made waves in the reproductive landscape. While the drug hasn’t made its way to retailers’ shelves yet, organization and subject matter experts anticipate its benefits and clinical time-saving abilities.
Over-the-Counter Birth Control Pills
On July 13, 2023, the US Food and Drug Administration (FDA) made a landmark change by approving Opill, a progestin-only oral contraceptive pill, for over-the-counter (OTC) sale. A year earlier, in July 2022, HRA Pharma, which manufactures the progestin-only pill, applied for OTC approval with the FDA.
Progestin-only hormonal birth control pills, sometimes called mini-pill, have been used across the US as a prescription drug over the past 50 years. These pills use progestin, a synthetic version of naturally occurring progesterone, to prevent ovulation and thicken cervical mucus to minimize the likelihood of implantation. Unlike combination pills, Opill doesn’t have estrogen, making it less likely to cause side effects, like blood clots, than combination birth control pills.
The submission was supported by multiple medical organizations, including the American Medical Association (AMA) and the American College of Obstetricians and Gynecologists (ACOG). According to IBIS Reproductive Health, the decision to make OTC birth control available was supported by decades of research and advocacy from healthcare professionals and members of the Free the Pill coalition.
Moreover, a joint FDA advisory panel comprised of individuals from the Nonprescription Drugs Advisory Committee (NDAC) and the Obstetrics, Reproductive, and Urologic Drug Advisory Committee (ORUDAC) unanimously voted, favoring FDA approval of the OTC birth control pill.
Prescription Birth Control Pills
Nationwide Children’s provides the pathway below as a guide for prescribing contraceptives.
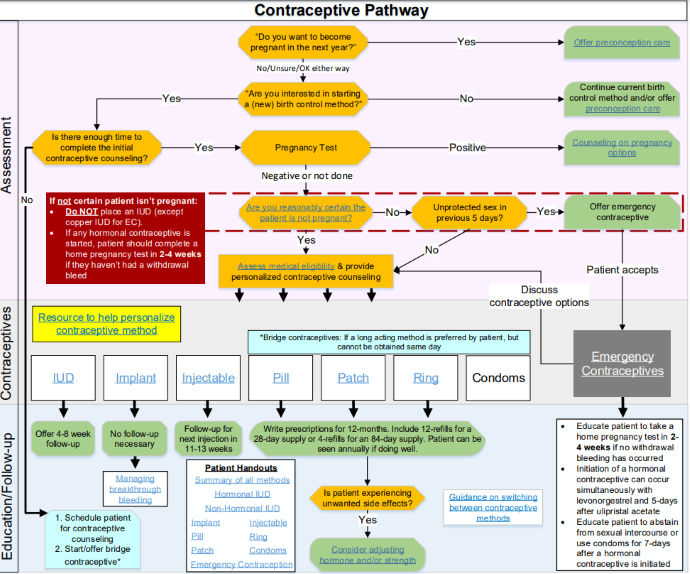
To get a prescription from a provider, patients must visit a doctor’s office, health clinic, or other family planning organizations.
Data from the Guttmacher Institute reveals that 28 states and DC allow pharmacist-authorized prescriptions for contraception, with 27 states specifying the contraceptive methods that pharmacists can prescribe.
Other limitations include patient age restrictions from 12 states and mandatory primary care provider visits for refills in seven states. Although some jurisdictions have eased prescription protocols, allowing for pharmacist-authorized and online prescriptions, these alternatives only solve some barriers to care.
According to Planned Parenthood, getting a prescription birth control pill can be complicated. A gynecology visit to get a prescription for birth control can cost between $35 and $250 before the $0–50 charge for birth control. While many healthcare plans under the Affordable Care Act cover both control-related visits, some underinsured patients may bear this cost.
For example, pharmacist-authorized prescriptions still require a patient to pay for what can be a costly prescription. On the other hand, obtaining a prescription online can be dangerous as counterfeit drugs and prescription fraud can be a complication.
Comparing Prescription and Over-the-Counter Pills
Oral hormonal birth control pills are one of the most commonly used contraceptive methods. The newly approved OTC Opill is projected to have widespread impacts on patients, providers, and the healthcare system, as multiple other OTC drugs do.
The transition of a drug from prescription to over-the-counter approval can be a complicated process. The FDA has two pathways for OTC approval: a new drug application (NDA) and the OTC monograph process.
An OTC monograph can be initiated by the manufacturer or developer of the product or the FDA. When reviewing a drug for OTC approval, regulatory authorities consider benefit–risk comparisons, consumer-friendly labeling, and how it compares to existing prescription medicines.
In a white paper published by OTC Medicines, the Consumer Healthcare Products Association’s Clinical/Medical Committee explored the benefits of over-the-counter medicines and how they impact the healthcare system. These benefits may be expanded as new approval, such as OTC birth control, are implemented.
Patients
Access to reproductive health and family planning services can be a significant challenge for fertile patients and couples across the US. The US Department of Health and Human Services Office on Women’s Health notes that before the approval of the OTC oral hormonal contraceptive pill, the only contraceptive methods available without a prescription were male or female condoms, spermicide, contraceptive sponges, and emergency contraceptive methods known as the morning-after pill. However, these forms of birth control are not as effective as some hormonal methods.
Until now, access to most hormonal reproductive healthcare hinged on a patient’s ability to see a healthcare provider and get a prescription. However, CAP notes that nearly one-third of women seeking contraceptives are limited by multiple factors, including financial, transportation, and cultural or linguistic barriers.
With an over-the-counter version, contraceptive access will be significantly greater for all patient populations. Rather than going to a provider’s office, dealing with health insurance coverage, and taking all the steps patients must complete before getting their prescription, eligible patients can go to their local drug store or retailer and buy the pill.
OTC Medicines explained that there are also convenience values to OTC medicine. Conveniences may include location and access convenience, in addition to the convenience of having more choices. Often, convenience entices patients to seek care, making them less likely to deal with the outcomes of forgoing care.
In addition, OTC switches can contribute to widened treatment adoption. For example, the Consumer Healthcare Products Association (CHPA) estimates that patient use increased by 150–200% when nicotine replacement therapies became available OTC.
Cutting down the time and steps to treatment significantly, OTC hormonal birth control methods can help minimize the rate of unintended pregnancy and protect patients from other pregnancy complications.
Providers
According to the OTC Medicine white paper, clinical practice guidelines often recommend OTC medications. Data from the National Guideline Clearinghouse database revealed that many OTC medications or ingredients are recommended for several conditions, making treatments for certain conditions more accessible.
Beyond being able to prescribe OTC medications as a first-line treatment, OTC switches can significantly cut down on time, including examination, prescribing, medication education, and prescription filling time. Considering the ongoing clinician shortage — and the projected deficit of OB/GYNs based on reduced residency applications — minimizing extraneous work can open physicians up to care for patients with more severe health conditions.
Healthcare System
Claims from the OTC Medicine article note that OTC drugs can decrease treatment gaps in prescription medications. As the cost of prescription drugs continues to rise, the more affordable prices of OTC medications are enticing to consumers.
“OTC medications are not associated with the same barriers to use as prescription medications. Consequently, the treatment gap for a condition may decrease if an OTC therapy becomes available.” noted the white paper.
In a report published by Pfizer, the organization notes that OTC medicines can save consumers $5.2 billion yearly by promoting patient self-care and eliminating unnecessary doctor visits. The company also notes that for each dollar spent on OTC medications, the health system saves $6.50, with $1.60 in drug cost savings and $4.90 in clinician visit cost savings.
Access to contraception is a form of preventative healthcare, minimizing rates of unintended pregnancies and subsequent treatments or procedures. Whether a patient opts for termination or carries the pregnancy to term, an unintended pregnancy adds pressure to the healthcare system, increasing provider workloads and costing the system significantly more than effective contraception.
While Opill has yet to make its way into drug stores and Perrigo, the parent company of HRA Pharma, has yet to release pricing protocols, researchers and healthcare providers continue to speculate on the potential impact of OTC birth control. Regardless of personal or public opinion, analyzing the long-term clinical implications of OTC availability can contextualize and set precedents for future OTC approvals.






