
elenabs/istock via getty images
What Are Precision Medicine and Personalized Medicine?
Precision medicine, also known as personalized medicine, is a new frontier for healthcare combining genomics, big data analytics, and population health.
Since the beginning of recorded history, healthcare practitioners have striven to make their actions more effective for their patients by experimenting with different treatments, observing and sharing their results, and improving upon the efforts of previous generations.
Becoming more accurate, precise, proactive, and impactful for each individual that comes under their care has always been the goal of all clinicians, no matter how basic the tools at their disposal.
But now, modern physicians and scientists are now able to take this mission far, far beyond the reach of their ancestors with the help of electronic health records, genetic testing, big data analytics, and supercomputing – all the ingredients required to engage in what is quickly becoming truly precise and personalized medicine.
Precision medicine, also commonly referred to as personalized medicine, is one of the most promising approaches to tackling diseases that have thus far eluded effective treatments or cures. Cancer, neurodegenerative diseases, and rare genetic conditions take an enormous toll on individuals, families and societies as a whole.
Approximately 1.7 million new cancer cases were diagnosed in the United States in 2017. Around 600,000 deaths were expected during that year, according to the American Cancer Society. The Agency for Healthcare Research and Quality adds that the direct economic impact of cancer is around $80 billion per year – loss of productivity, wages, and caregiver needs sap billions more from the economy.
Many of these cancers are preventable through lifestyle changes, such as quitting tobacco, improving weight and diet, or reducing alcohol consumption, but many are also the result of predispositions to certain diseases inherited along ethnic, racial, or familial lines.
Neurological diseases, including Alzheimer’s disease, produce similarly dire impacts. Alzheimer’s is the 6th leading cause of death in the United States, and the Alzheimer’s Association predicts that 16 million individuals will be living with the disease by 2050.
Alzheimer’s and other forms of dementia cost the US approximately $259 billion in 2017. As the population ages and dementia cases increase in number, those costs will top $1.1 trillion by 2050.
The financial, clinical, and social imperatives for finding cures for these and other conditions have led to a surge in interest around precision medicine. With much more digital data at their disposal and the computing power to crunch the numbers, researchers are now eagerly uncovering new relationships between genes, drugs, and populations.
Fully unlocking the secrets of how an individual’s genetics impacts his or her likelihood of developing or surviving a particular condition would produce a fundamental revolution in the way providers approach the practice of medicine, moving the profession an incalculable distance from the basic experimentation of its prehistorical origins.
What is precision medicine?
According to the Precision Medicine Initiative, precision medicine is “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person.”
Physicians have, of course, tailored their recommendations to individual factors such as age and gender, patient preferences, mobility levels, community resources, preexisting conditions, and other mitigating circumstances for many, many years.
The difference between a traditional shrewd assessment of a complex situation and true “precision medicine” is the degree of reliance on data – especially genomic data – to make decisions about specific treatment paths that may be more or less effective for the individual at hand.
Genomic data is a relatively new addition to the physician’s toolkit, and researchers have barely begun to scratch the surface of what revolutionary insights are likely hidden in the human genetic code.
Genetic testing is getting quicker and cheaper, offering researchers the opportunity to collect larger volumes of data from more diverse patient groups.
By combining this data with clinical, pharmaceutical, and socioeconomic information, and then applying analytics to these integrated datasets, researchers and providers can observe patterns in the effectiveness of particular treatments and identify the genetic variations that may be correlated with success or failure.
Clinical trials can then be used to test and validate these hypotheses. If the results meet rigorous scientific standards, they may support future best practices or clinical guidelines for the treatment of specific conditions integrated into advanced clinical decision support systems.
How does personalized medicine differ from precision medicine?
Many stakeholders are starting to prefer the term “precision medicine” instead of “personalized medicine” to describe the use of data and genomics to tailor treatments to specific groups.
The National Research Council (NRC) has expressed concern that “personalized medicine” may be misconstrued to mean that completely individualized treatments are available for every unique patient, which is not the case.
In its 2011 report Towards Precision Medicine, the NRC states:
Precision medicine refers to the tailoring of medical treatment to the individual characteristics of each patient. It does not literally mean the creation of drugs or medical devices that are unique to a patient, but rather the ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease…or in their response to a specific treatment. Although the term “personalized medicine” is also used to convey this meaning, that term is sometimes misinterpreted as implying that unique treatments can be designed for each individual. It should be emphasized that in “precision medicine,” the word “precision” is being used in a colloquial sense, to mean both “accurate” and “precise.”
“Personalized medicine” has been falling out of favor since 2015, when President Barack Obama introduced the national Precision Medicine Initiative in his State of the Union speech, explained Muin J. Khoury, Director, Office of Public Health Genomics, Centers for Disease Control and Prevention, in a 2016 blog post.
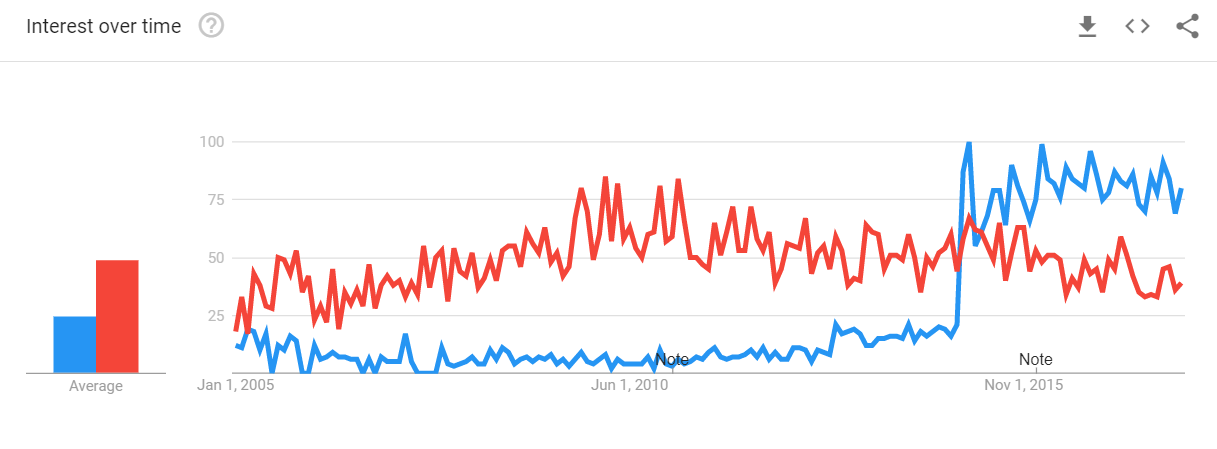
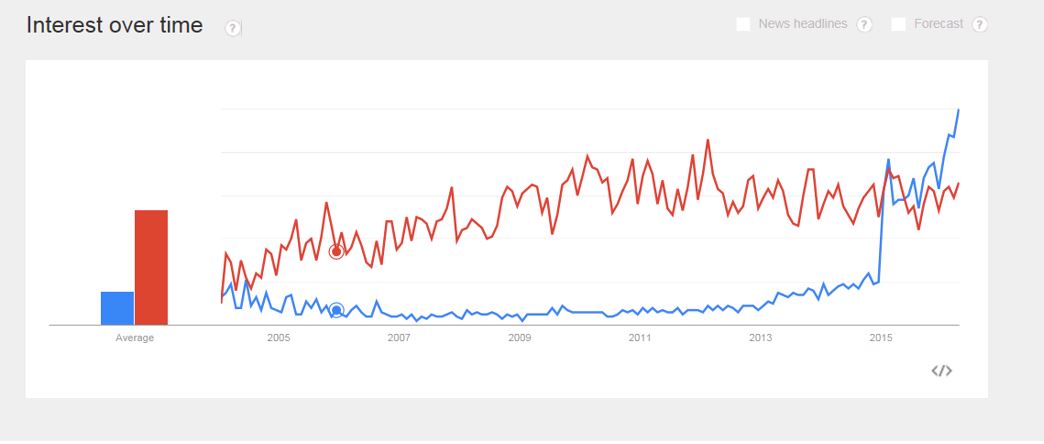
“Precision medicine” has overtaken “personalized medicine” as the preferred search term online, and the number of academic journal articles using “precision” instead of “personalized has exploded.
The difference in search popularity is even more marked at the beginning of 2018, with “precision medicine” firmly the favorite among Google users in the United States.
The growing popularity of “precision medicine” over “personalized medicine” may be a good thing, Khoury said, as targeted therapies become more deeply integrated into everyday clinical care, requiring patient education and tempered expectations.
“To be sure, precision medicine and personalized medicine are highly related and genomics plays a big role in both,” said Khoury. “However, even highly personalized information may or may not lead to improved health outcomes. Moreover, precision medicine approaches may lead to non-personalized interventions that can be used population-wide.”
Direct-to-consumer (DTC) genetic testing kits, such as those offered by 23andMe or AncestryDNA, are an example of the divide between personalization and precision, he continued.
These commercial products can deliver very personalized information, but may not be particularly precise or actionable when it comes to an individual’s health status or chances of developing specific conditions.
“In many ways, DTC genetic tests are the opposite of precision medicine,” observed Khoury. “In spite of their deceptive appeal, they ‘deliver uncertain information and create patient expectations that may align poorly with evidence; clinical priorities; or, in some cases, the patient’s best interests.’”
Using “precision medicine” instead of “personalized medicine” as the latter continues to slide out of favor may help to ensure clarity for patients who are interested in learning more about tailored care.
Interest in Genetic Testing Brings Precision Medicine Challenges
Artificial Intelligence, Genomics Combo Boosts Precision Medicine
Defining key terms within precision medicine
Many of the scientific terms used to describe the progress and challenges of precision medicine require highly specialized knowledge to fully understand, which can be an obstacle for providers who wish to educate patients about precision medicine, its opportunities, and its limitations.
While most people are familiar with the concept of DNA and its basic role in many diseases, painting a fuller picture of how and why targeted therapies work may involve providing explanations of several of the following terms.
Genomics
The genome is an organism’s complete set of genetic material. DNA includes coding regions, or genes, that govern the function of proteins, as well as non-coding sequences that perform regulatory functions. Genomics is the study of these materials.
Genetics
A gene is a subset of the genome that codes for a molecule that has a specific function, such as governing a person’s eye color, blood type, or predisposition for certain diseases. Genes can acquire mutations when passed along through families, resulting in inherited conditions. Variations in an individual’s phenotype, or the sum of its observable physical or behavioral characteristics, are due part to how individual genes combine to produce those traits.
Researchers are still discovering incredibly complex relationships between genes and diseases. The rapid advance of genetic research in the early years of the 21st century has been supported by initiatives like the Human Genome Project, which produced a composite genetic sequence that is freely available to the public. Scientists can compare cancer cells, for example, with this data to understand where and how specific mutations occur and what their impacts might be.
Genetic sequencing
Genetic sequencing, or DNA sequencing, is the process of determining the order of the four chemical building blocks of DNA (adenine, guanine, cytosine, and thymine) for an individual organism. The order of these chemicals in each strand of DNA dictates the type of genetic information included in a segment of DNA.
Researchers can identify which sections of a DNA molecule contain genes and which include regulatory information, allowing them to pinpoint differences between individuals with certain traits and those without. The human genome contains around 3 billion pairs of the four chemical bases of DNA, which all together provide the “instruction manual” for a living organism.

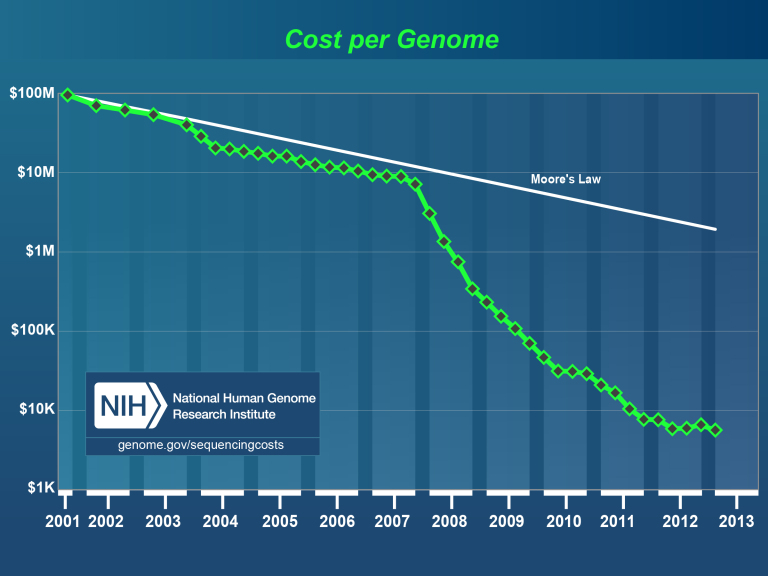
Since its start in the early 1970s, genetic sequencing has become so quick and inexpensive that healthcare providers can routinely order genetic testing for patients suspected of having conditions with a genetic component.
The falling prices and rising speed of these tests has been a significant catalyst for precision medicine. Interest in genetic testing has skyrocketed in recent years, with 90 percent of patients in one recent survey expressing some level of curiosity about receiving a genetic test.
Next-generation sequencing
Next-generation (next-gen) sequencing is a collection of techniques that have further enhanced the speed and detail of genetic sequencing.
Instead of sequencing an individual’s entire genetic code from scratch every time, next-gen techniques sequence fragments of an individual’s DNA, called “reads,” and then use algorithms to compare the results to a DNA library to fill in the gaps. Any differences or mutations can be identified during the process.
Next-gen sequencing allows laboratories to complete the process more quickly, helping to meet the growing demand for their services. The techniques are being developed and refined very rapidly as life science companies rush to provide researchers and healthcare organizations with one of the essential tools for precision care.
CRISPR
CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats), are part of an organism’s bacterial defense system, first discovered by Francisco Mojica, a scientist at the University of Alicante in Spain.
They “consist of repeating sequences of genetic code, interrupted by ‘spacer’ sequences – remnants of genetic code from past invaders. The system serves as a genetic memory that helps the cell detect and destroy invaders (called ‘bacteriophage’) when they return,” says the Broad Institute.
CRISPRs form the basis for CRISPR-Cas9, a gene editing technology that allows researchers to target specific stretches of genetic code and alter the DNA within them.
This futuristic capability may allow scientists to correct damaging mutations or susceptibilities to diseases in living organisms without side effects from traditional pharmaceutical therapies – and even rectify genetic conditions for which no pharmaceutical option exists.
Pharmacogenetics
Pharmacogenetics is the study of how genetic differences influence the metabolic pathways of drugs, or how individuals respond to specific pharmaceutical interventions based on their unique genetic makeup. This science forms the basis of many precision medicine efforts, such as choosing individualized drug combinations to attack a specific type of cancer.
For example, the drug Tamoxifen is widely used to treat breast cancer or prevent a recurrence, but up to 10 percent of individuals have a version of a gene that results in resistance to its positive effects.
Before prescribing the drug, providers can order a genetic test to see whether the individual has a genetic makeup that would reduce the effectiveness of the drug.
Pharmacogenetics (often used interchangeably with pharmacogenomics) is also important for drug safety research and identifying potential negative drug-drug or drug-gene interactions.
Biomarkers
Biomarkers are widely used across all forms of clinical practice to indicate that a disease, infection, toxicity, or other process is taking place within an organism.
Biomarkers may include lead levels in the blood, antibodies after an infection, thyroid hormone levels, or prostate specific antigen (PSA), as well as molecular signatures can indicate whether a patient is likely to respond to certain advanced therapies.
The exploration of biomarkers for identifying and treating cancer and similar conditions with genetic components is one of the main branches of precision medicine research.
Biobanking
In many respects, genomic research is at its most useful when conducted at scale. In order to identify patterns in populations in a reliable and repeatable manner, researchers must have access to large volumes of patient data.
In addition, researchers investigating rare genetic conditions or those targeting therapies to very specific populations will only be able to find suitable candidates for clinical trials if they have as many individuals as possible to choose from.
Biobanking, or the act of collecting and storing samples of DNA from groups of individuals (typically blood, saliva, and/or urine), allows researchers to access larger pools of potential subjects who have already signaled a willingness to participate in studies or trials.
Biobanks have proliferated in recent years as healthcare provider systems and government agencies recognize the need to have as much data available for research as possible.
Large health systems such as Geisinger Health System, Northwell Health, and Kaiser Permanente have established their own biobanks to support their research communities, while the Department of Veterans Affairs currently oversees one of the largest biobanks in the country.
The All of Us research initiative, discussed further below, has also launched multiple efforts to kick off its enrollment processes with a special emphasis on collecting data from traditionally underserved communities and ethnically diverse participants.
70% of Orgs Planning Precision Medicine Will Deploy Within 2 Years
Pharma, Tec Companies Set to Restructure Healthcare Big Data Market
The Precision Medicine Initiative
2015 was a pivotal year for precision medicine as former President Obama launched the Precision Medicine Initiative (PMI) during that year’s State of the Union address.
The project, which received bipartisan support at the time, was designed to jumpstart research across the public and private sectors while encouraging everyday patients and their families to get involved in sharing their data and participating in clinical trials.
“I want the country that eliminated polio and mapped the human genome to lead a new era of medicine – one that delivers the right treatment at the right time,” Obama said at the time. “In some patients with cystic fibrosis, this approach has reversed a disease once thought unstoppable.”
“Tonight, I’m launching a new Precision Medicine Initiative to bring us closer to curing diseases like cancer and diabetes – and to give all of us access to the personalized information we need to keep ourselves and our families healthier.”
The centerpiece of the initiative was the PMI Cohort, now called the All of Us Research Program. With the goal of recruiting one million Americans to participate, All of Us will eventually provide researchers with access to huge volumes of data to fuel their work.
"Researchers will use data from the program to learn more about how individual differences in lifestyle, environment and biological make-up can influence health and disease," said the National Institutes of Health (NIH) in 2017.
"By taking part, people will be able to learn more about their own health and contribute to an effort that will advance the health of generations to come.”
Socioeconomic, racial, and ethnic diversity are high priorities for the program, which has started to recruit community organizations to spread the word among their participants.
“Community partners are integral to All of Us,” said Eric Dishman, Director of the All of Us program. “This first-of-its-kind program seeks to include people from all walks of life, and these community partner awardees were selected to help achieve that goal.”
The NIH is primarily responsible for overseeing the All of Us project, but is working with conjunction with the FDA, HHS, the Department of Veterans Affairs, Department of Defense, the National Cancer Institute (NCI) within the NIH, and a number of healthcare industry stakeholders to facilitate the Precision Medicine Initiative as a whole.
On a similar theme as the PMI, former Vice President Joe Biden oversaw the launch of the national Cancer Moonshot, supported by funds from the landmark 21st Century Cures Act passed in late 2016.
The Cancer Moonshot aims to use the growing wealth of available healthcare big data to foster breakthroughs in research and cancer treatment. The collaborative effort is bringing together both public and private organizations to support research and develop new treatments for deadly diseases.
“Everywhere I travelled [meeting with researchers and patients], I was told that data are key, and we have an unprecedented amount and diversity of data being generated daily through genomics, family history records, lifestyle measurements, and treatment outcomes,” said Biden in a 2016 report on the future direction and challenges of the initiative.
“We now have the capability to realize the promise of all of these data because of advances in super computing power. Researchers can analyze enormously complex and large amounts of data to find answers we couldn’t just five years ago.”
CMS may be planning to add even more data to the pool by considering coverage options for a new FDA-approved genetic test that can identify key biomarkers for multiple cancers.
The FoundationONe CDx diagnostic test can flag mutations in 324 genes and two genomic signatures in solid tumors.
The test can also suggest which of 15 existing FDA-approved cancer treatments would be most applicable to patients with one of five tumor types, including non-small cell lung cancer, melanoma, breast cancer, colorectal cancer, and ovarian cancer.
Expanding the availability of such a test to Medicare beneficiaries could rapidly accelerate the goals of both the Cancer Moonshot and the larger Precision Medicine Initiative.
Top 5 Basics to Know about the Precision Medicine Initiative
Mayo Gets $142M to Create Precision Medicine Initiative Biobank
How the private sector is pursuing precision medicine
Federal investment is only the beginning of the healthcare system’s involvement in furthering precision medicine. The private sector is rapidly embracing similar goals, which align well with existing value-based reimbursement and population health management initiatives.
Academia has been particularly active in the precision medicine ecosystem, combining computer science and engineering breakthroughs with laboratory work and clinical discovery. Similarly, pharmaceutical companies angling to expand their drug portfolios are heavily investing in research and collaborations with healthcare providers and others.
Even Silicon Valley figures like Bill Gates and Mark Zuckerberg are addressing the urgent need for precision medicine development: Gates recently announced a $50 million investment in data-driven Alzheimer’s research, while Zuckerberg and his wife, Dr. Priscilla Chan, made a $10 million donation to the UCSF Institute for Computational Health Sciences to explore how to best reuse available datasets to produce new insights.
Many activities revolve around collaborations and partnerships between likeminded organizations looking to pool their computing power and share insights across research networks. Some notable examples include:
Geisinger Health System has launched its own National Precision Health Initiative which will leverage the data from 160,000 volunteer patients held in its MyCode Community Health Initiative biobank. The initiative will focus on developing strategic partnerships and new ventures while harnessing the existing resources within the Geisinger network.
Eleven biopharmaceutical companies and the National Institutes of Health are working together on the $215 million Partnership for Accelerating Cancer Therapies (PACT) program, which is an offshoot of the Cancer Moonshot. The public-private coalition will work to identify and validate biomarkers that could lead to immunotherapies for a variety of cancers.
Indiana University (IU) and the Regenstreif Institute have given technology company LifeOmic the rights to access research and faculty resources in exchange for a minority stake in the company. The three organizations will develop a “data commons” for precision medicine research that hopes to include genetic and clinical data on millions of patients.
Five campuses within the University of California system have partnered to form a big data analytics and precision medicine research network that plans to reduce the social, economic, and clinical burdens of cancer on state residents. UC Davis, UC Irvine, UCLA, UC San Diego and UCSF will combine precision medicine with population health management to better understand and address the impacts of cancer on California citizens.
GE Healthcare and Roche Diagnostics recently announced a long-term partnership to develop a big data analytics and clinical decision support platform to support precision medicine research. The collaboration will focus on integrating in-vivo and in-vitro data, electronic health records, clinical guidelines, and real-time monitoring data to tailor treatments to individual needs.
With support from Memorial Sloan Kettering Cancer Center, The Broad Institute at MIT, and Harvard, IBM Watson Health and Quest Diagnostics have launched a new service that plans to deliver genomics-rich clinical decision support to the point of care. Clinicians will be able to send solid tumor biopsies to Quest Diagnostics, which will feed the data into IBM Watson Health’s genomics analytics tools to produce treatment suggestions.
These efforts, and many more like them in labs, academic institutions, and healthcare systems across the country, have the potential to dramatically accelerate the availability of highly targeted therapies for some of the most intractable and devastating diseases.
Patients who previously had few options or low odds of survival may soon find hope as both the public and private sectors uncover more insights into the complex interplay between genetics and diseases.






